Search API
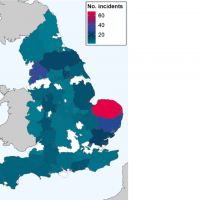
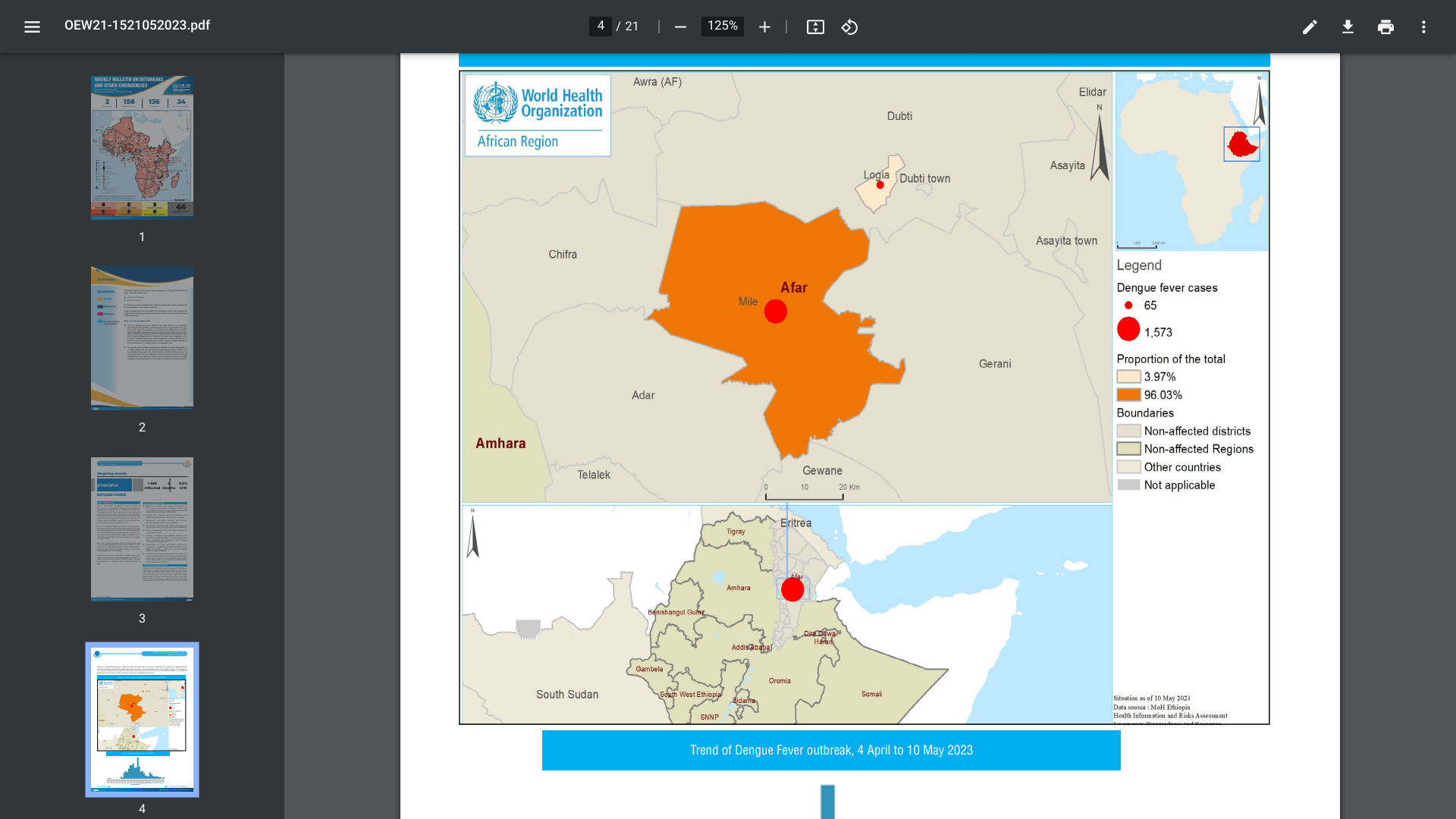
The World Health Organization (WHO) Africa Region recently reported two districts (Logia and Mille) of the Afar region in north-eastern Ethiopia are experiencing a Dengue fever outbreak.
The Mille district has reported the most dengue cases (96%).
As of May 10, 2023, a total of 1 638 suspected and confirmed dengue cases and nine associated deaths (CFR 0.5%) have been reported.
Of the nine suspected deaths reported, four occurred at the health facility and five at the community level.
Ethiopia has had nearly annual outbreaks since 2013, devastating an already fragmented health system, says the WHO. Furthermore, dengue is a vaccine-preventable disease, with two vaccines available in 2023.
Other dengue outbreaks in 2023 are posted at Vax-Before-Travel.
The U.S. CDC Morbidity and Mortality Weekly Report (MMWR) Podcast Briefing, published today, offers an overview of the latest scientific information regarding mpox vaccinations.
This podcast discusses three MMWR reports as of the week of May 15, 2023.
First, although the number of mpox cases has decreased since the peak of the U.S. outbreak in August 2022, the risk for future outbreaks remains.
And clinicians need to be alert for new cases, and people at risk should continue to take prevention measures.
Second, a new report looking at data from 12 U.S. jurisdictions shows Bavarian Nordic JYNNEOS® (MVA-BN) vaccine effectively prevents mpox in people at high risk for mpox.
Third, a study of mpox patients in New York provides evidence that the JYNNEOS vaccine is highly effective against mpox.
However, the CDC and other health agencies in France, South Korea, and Spain previously reported various mpox breakthrough cases in 2023.

EL PAIS recently reported two patients were diagnosed for the second time with mpox in Spain.
On May 26, 2023, the Vall d'Hebron (Barcelona) and Ramón y Cajal (Madrid) hospitals confirmed two patients had been reinfected with mpox.
EL PAIS reported these are the first mpox reinfection cases in Spain.
According to the Carlos III Health Institute, there have been 56 mpox cases in Spain since the beginning of 2023.
Previous reports in 2023 confirmed secondary mpox cases in Chicago, Il, Paris, France, and Seoul, South Korea.

Immorna today announced that the first subject had been dosed in the Company's First-In-Human Phase 1 multi-center study of JCXH-105, a self-replicating RNA (srRNA) vaccine being developed for the prevention of Shingles.
The U.S. Food and Drug Administration (FDA) cleared its investigational new drug application on January 9, 2023, to conduct a Phase 1 multi-center study of JCXH-105.
NgocDiep Le, M.D., Ph.D., Global Chief Medical Officer of Immorna, commented in a press release on May 30, 2023, "If proven successful in clinical studies, JCXH-105 may become a valuable alternative to current standard-of-care to meet the large world-wide medical need for Shingles prevention."
"Due to its self-replicating nature, JCXH-105 may be effective at a significantly reduced dose level compared to non-replicating conventional mRNA vaccines and thereby may cause less reactogenicity and substantially reduce the cost of production."
"In addition, due to the synthetic nature of all JCXH-105 vaccine components, there are no raw material limitations or production bottlenecks."
This Phase 1 study is a randomized, double-blinded, multi-center, active-controlled study to assess the safety, immunogenicity, and determine the Recommended Phase 2 Dose for JCXH-105 for seniors.
In this study, JCXH-105 will be compared to GSK's U.S. FDA-approved Shingrix® vaccine.
Other shingles vaccine development news is posted by Precision Vaccinations.

Valneva SE today announced filing a regulatory application with Health Canada for marketing approval of the single-shot chikungunya vaccine candidate, VLA1553.
If accepted by Health Canada, VLA1553 would become available for persons aged 18 years and above.
VLA1553 is currently the only chikungunya vaccine candidate worldwide for which regulatory review processes are underway. It could become the first licensed chikungunya vaccine to address this unmet medical need if approved.
A Biologic License Application is currently under priority review by the U.S. Food and Drug Administration with a Prescription Drug User Fee Act review goal date at the end of August 2023.
VLA1553 received FDA Fast Track and Breakthrough Therapy designations in 2018 and 2021, respectively. The program was also granted PRIority MEdicine designation by the European Medicines Agency in 2020.
Furthermore, Valneva plans to make regulatory submissions for VLA1553 in Europe in the second half of 2023.
Juan Carlos Jaramillo, MD, Chief Medical Officer of Valneva, commented in a press release on May 30, 2023, "Chikungunya represents a major threat for people traveling to or living in areas where chikungunya virus and the mosquitos that transmit it are present, including popular destinations for U.S. and Canadian travelers."
"No vaccine or specific treatments are currently available for this debilitating disease, and we will continue to work diligently to bring VLA1553 to different territories as soon as possible."
Chikungunya is a mosquito-borne viral disease caused by the chikungunya virus, transmitted by Aedes mosquitoes.
An infection leads to symptomatic disease in 72-92% of humans after four to seven days following the mosquito bite. While mortality is low, morbidity is high.
Beginning in 2014, chikungunya virus disease cases were reported among U.S. travelers returning from affected areas in the Americas, and local transmission was identified in Florida, Texas, Puerto Rico, and the U.S. Virgin Islands, says the U.S. CDC.
The high-risk areas of infection for travelers included the Americas, parts of Africa, and Southeast Asia, and the virus has spread to more than 110 countries.

The U.S. Food and Drug Administration (FDA) recently issued a draft guidance titled: Diabetes Mellitus: Efficacy Endpoints for Clinical Trials Investigating Antidiabetic Drugs and Biological Products Guidance for Industry.
This draft guidance provides a 15-year update to the FDA's previous recommendations on efficacy endpoints for such products.
"Diabetes is a common disease that affects nearly 40 million people in the U.S. and is projected to affect more in the coming years. Therefore, the need for more antidiabetic treatment options is clear," said Lisa Yanoff, M.D., deputy director of the Office of Cardiology, Hematology, Endocrinology, and Nephrology in the FDA's Center for Drug Evaluation and Research, in a press release on May 26, 2023.
The draft guidance outlines the FDA's general recommendations around evaluating the efficacy of antidiabetic drugs for adults and children with type 1 and/or type 2 diabetes.
As of May 29, 2023, the FDA has not approved a diabetes preventive vaccine.

S.K. Chemicals' SKYCovion COVID-19 vaccine has been authorized by the U.K.'s Medicines and Healthcare Products Regulatory Agency (MHRA).
This authorization is for use as a primary vaccination in those aged 18 and over. Decisions on which COVID-19 vaccines are deployed in the U.K. are taken by the Joint Committee on Vaccination and Immunisation.
As of May 26, 2023, it becomes the 8th COVID-19 vaccine authorized by the U.K.
The SKYCovion vaccine combines a part of the SARS-CoV-2 virus spike protein with an 'adjuvant' – an additional ingredient designed to trigger a more robust immune response. It is given as two injections, four weeks apart.