Search API
Nykode Therapeutics ASA today announced that it had expanded the clinical collaboration and supply agreement with Roche to cover the evaluation of VB10.16, an off-the-shelf therapeutic cancer vaccine candidate in development for the treatment of human papillomavirus type 16 (HPV16)-positive cancers.
This collaboration, unveiled on June 1, 2023, is evaluating the combination with Roche’s cancer immunotherapy atezolizumab in patients with advanced cervical cancer who have progressed on pembrolizumab plus chemotherapy +/- bevacizumab as first-line treatment.
The VB-C-04 clinical trial is expected to be initiated in the U.S. in the fourth quarter of 2023 with registrational intent, which provides a potential fast-to-market path.
“We look forward to expanding our collaboration with Roche to bring the combination of VB10.16 and atezolizumab to cervical cancer patients with limited treatment options. Cervical cancer has a poor prognosis, and the recent positive clinical data from VB-C-02 has strengthened our commitment to contribute to a well-tolerated treatment that can potentially prolong the life of these women. The trial provides a potential fast path to market for VB10.16,” commented Michael Engsig, CEO of Nykode Therapeutics, in a press release.
Precision Vaccinations post other cervical cancer vaccine updates.

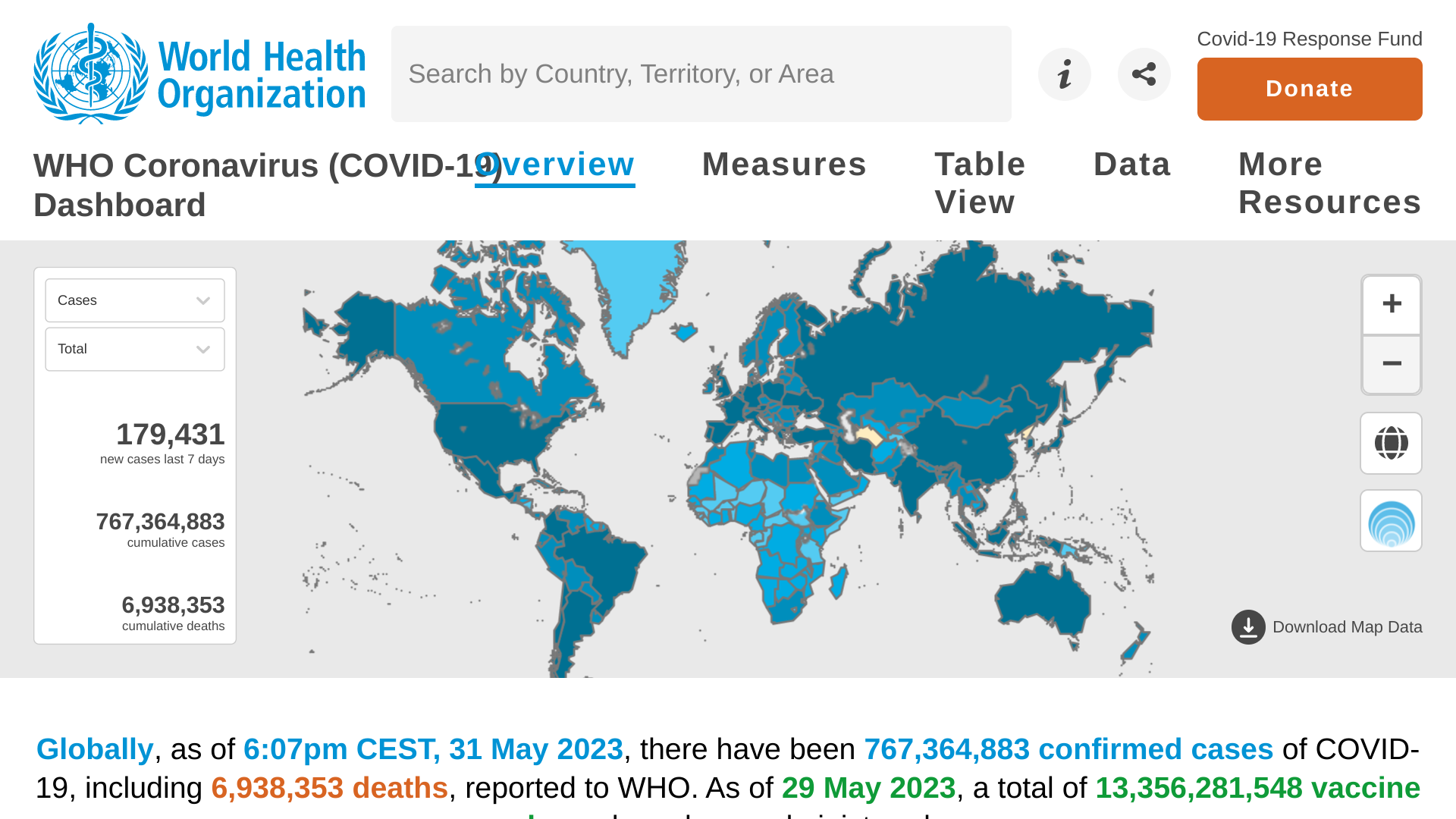
Globally, nearly two million new COVID-19 cases and over 12,000 related fatalities were reported in the last 28 days, a decrease of 30% and 39%, respectively, compared to the previous 28 days, reported the World Health Organization (WHO).
As of June 1, 2023, the situation is mixed at the regional level, with increases in reported cases seen in the Western Pacific Region and the African Region and decreases in related fatalities in all six WHO regions.
During this 28-day reporting period, 150 of 243 (62%) countries and territories reported at least one COVID-19 case.
The European Region had the highest proportion of countries reporting data on new hospitalizations (19 countries; 31%).
Globally, XBB.1.5 has been reported from 115 countries since the variant emerged.
Florida Health recently published an updated Mosquito-Borne Disease Surveillance report that indicates there were three new cases of dengue confirmed last week in persons that had international travel.
As of May 27, 2023, seventy-seven travel-associated dengue cases have been reported in Florida during week #21.
And there have been two cases of locally acquired dengue in 2023.
As of June 1, 2023, the U.S. CDC reported 129 dengue cases in States and 256 dengue cases in U.S. Territories this year.
Other dengue outbreaks are posted at Vax-Before-Travel.

The journal PLOS One reported every 75 seconds, a child under five dies of malaria, and children bear the highest burden of malaria in Sub-Saharan Africa (SSA).
On May 31, 2023, the pooled prevalence of malaria among children aged 6–59 months was 27.41% (95% CI: 17.94%-36.88%) in SSA.
Infection rates range from 5.04% in Senegal to 62.57% in Sierra Leone.
This study revealed that older under-five children living in large families with low incomes in rural areas are most vulnerable to malaria infection.
To notify international travelers of their potential health risks, the U.S. CDC issued a Level 2 travel advisory focused on malaria outbreaks in Costa Rica.
While there are malaria vaccines available in June 2023, the deployment has yet to reach critical mass in SSA.
YS Biopharma Co., Ltd. today announced that its novel PIKA Rabies Vaccine was granted Phase 3 clinical trial approval from the Food and Drug Administration of the Philippines.
The Phase 3 clinical trial, which is planned to commence later in 2023, will include approximately 4,500 subjects in the Philippines, Singapore, and Pakistan.
The PIKA Rabies Vaccine has the potential to become the first accelerated three-visit one-week regimen, superior to the currently available vaccine with a five-visit one-month or three-visit three-week regimen.
The US FDA granted orphan-drug designation for preventing rabies infection, including post-exposure prophylaxis (PEP) for rabies.
The PIKA Rabies Vaccine is powered by YS Biopharma's proprietary PIKA adjuvant technology to induce accelerated immunity and produce a higher immune response.
Pending the successful completion of Phase 3 trials, the Company plans to launch the sales and marketing of the Vaccine in North America, as well as in countries throughout Asia, Africa, Europe, the Middle East, and Central and South America.
Rabies is a vaccine-preventable, zoonotic, viral disease affecting the central nervous system. It has a case-fatality rate of almost 100%.
According to the World Health Organization, about 59,000 people die of rabies annually in over 150 countries.
Over 30% of rabies victims are children.
The U.S. Centers for Disease Control and Prevention updated its recommendations for rabies preexposure prophylaxis for humans in June 2022, replacing the three-dose vaccination schedule with a two-dose program, intending to protect for about least three years.
As of June 1, 2023, there are various rabies vaccines approved.

Pfizer Inc. today announced that the U.S. Food and Drug Administration (FDA) had approved ABRYSVO™, the company’s bivalent Respiratory Syncytial Virus (RSV) prefusion F (RSVpreF) vaccine.
This FDA approval on May 31, 2023, prevents lower respiratory tract disease caused by RSV in individuals 60 years and older.
On May 3, 2023, the FDA approved the initial RSV vaccine, Arexvy™, which GSK produces.
According to statements, both RSV vaccines could be available for seniors in late 2023.
Other RSV vaccine candidates, including vaccines for pregnant women, are conducting late-stage clinical trials as of June 1, 2023.
The U.S. Centers for Disease Control and Prevention published a report on April 7, 2023, that indicated the 2022–23 RSV season started later than the 2021–22 season but earlier than the prepandemic seasons, suggesting a return toward prepandemic seasonality.
A study published by the Journal of Infectious Diseases determined that RSV-related fatalities in infants <1 year peaked at one month of age.
Over the 20-year study period, RSV, bronchiolitis, and influenza were listed as the underlying causes of death on 932, 1,046, and 52,293 death certificates, respectively.
Over 95% of these infections in children occur in low- and middle-income countries outside the U.S.

Southern Africa is again weathering a season of cholera, with six countries in the region recording outbreaks in 2023, reported Derick Matsengarwodzi with GAVI.
As of May 25, 2023, Malawi is the worst affected, recording 36,943 cases and 1,210 associated deaths between March 2022 and February 2023, according to World Health Organization (WHO).
So far this year, Malawi has received three shipments of oral cholera vaccine (OCV) in response to applications to the Gavi-supported stockpile established in 2013.
The latest 1.4 million OCV dose shipment arrived in Lilongwe in April 2023.
On May 22, 2023, Gavi published a roadmap outlining critical actions needed to ensure the supply of OCV can meet growing demand from countries.
The roadmap describes how these organizations, manufacturers, and countries can work together towards ensuring global OCV supply can support large-scale preventive vaccination by 2026.
"Cholera vaccines have steadily become more available over the past decade, meeting rising country demand," said Dr. Derrick Sim, Managing Director for Vaccine Markets and Health Security at Gavi, in a media release.
"As a result, the good news is we have doses to meet all emergency demand despite the rise in outbreaks, which is expected to continue. But this trend underscores the increasing importance of preventing outbreaks before they occur."
"The ultimate solution to both sustainable OCV supply and cholera control lies in our collective ability to step up our efforts on prevention programs."
As of May 31, 2023, various cholera vaccines have been approved but remain in limited supply.
Note: The U.S. CDC recently issued an Alert Level 2, Practice Enhanced Precautions, regarding polio outbreaks, which includes Malawi.
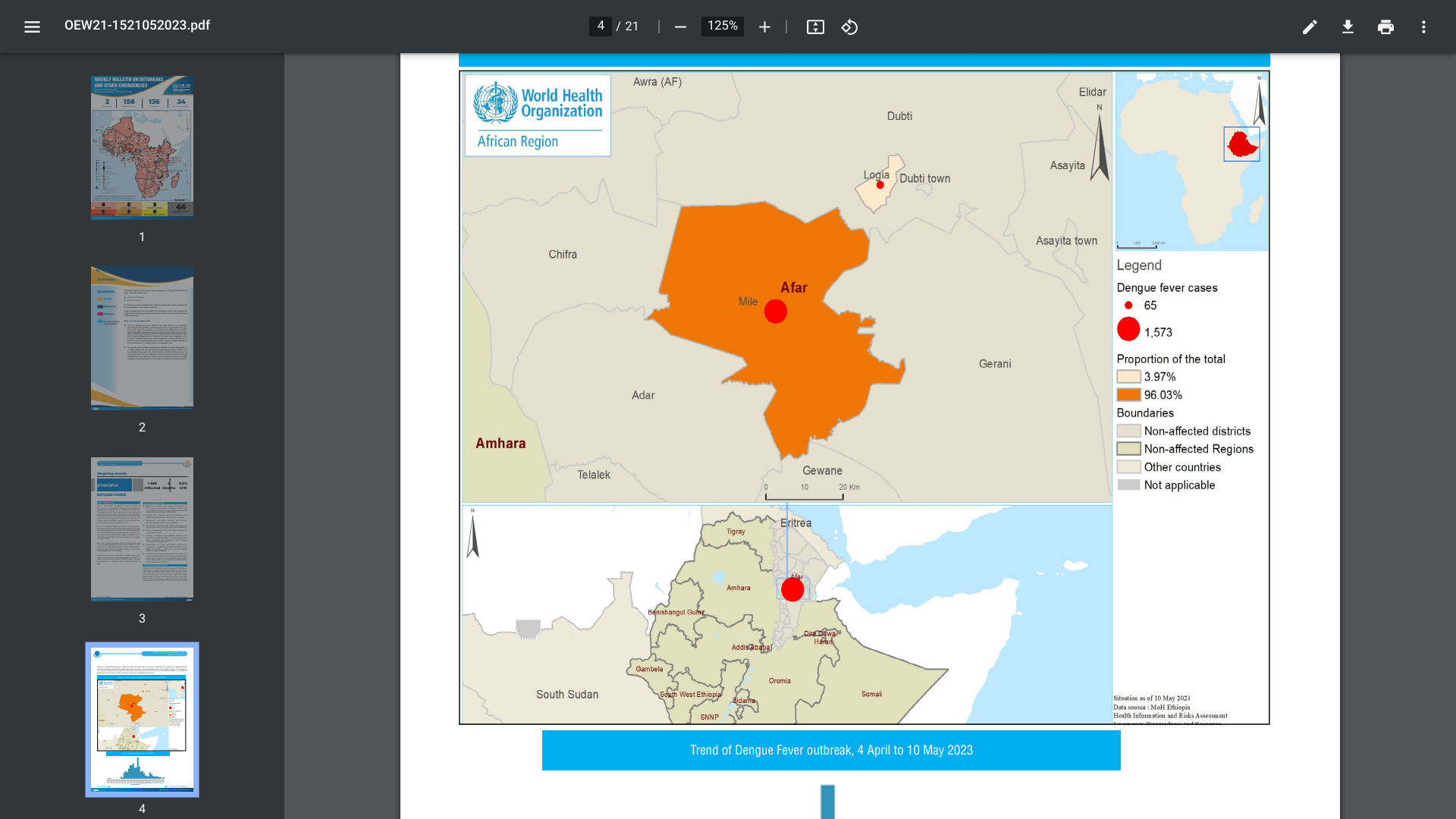
The World Health Organization (WHO) Africa Region recently reported two districts (Logia and Mille) of the Afar region in north-eastern Ethiopia are experiencing a Dengue fever outbreak.
The Mille district has reported the most dengue cases (96%).
As of May 10, 2023, a total of 1 638 suspected and confirmed dengue cases and nine associated deaths (CFR 0.5%) have been reported.
Of the nine suspected deaths reported, four occurred at the health facility and five at the community level.
Ethiopia has had nearly annual outbreaks since 2013, devastating an already fragmented health system, says the WHO. Furthermore, dengue is a vaccine-preventable disease, with two vaccines available in 2023.
Other dengue outbreaks in 2023 are posted at Vax-Before-Travel.