Search API
The U.S. Fish and Wildlife Service recently reported all vultures that received a highly pathogenic avian influenza (HPAI) vaccine candidate as part of the initial clinical trial on May 16, 2023, appears to be in good health with no vaccine site reactions.
As of June 2, 2023, the second vaccine dose will be administered next week.
Depending on these results, the second step would be to implement the vaccine trial on 25 captive California condors. Any vaccination of condors will be administered by a state-licensed veterinarian.
Since this HAPI outbreak was confirmed in 2023, 21 condors have died.
The California condor, with a wingspan of 9.5 feet and weighing up to 25 pounds, is the largest land bird in North America.
Previously, the United States Department of Agriculture's Agricultural Research Service (APHIS) researchers confirmed on April 14, 2023, they were testing several vaccine candidates.
The authorized vaccine is a killed, inactivated product conditionally licensed by APHIS' Center for Veterinary Biologics in 2016.
This vaccine has not previously been tested against this strain of the HAPI virus in California Condors. Depending on the results of this trial, the second step would be to implement the trial on 25 captive California condors.
This vaccine candidate is targeted at birds, not humans.
The U.S. government has already approved a bird flu vaccine for people and continues to invest in newer avian influenza vaccine candidates.

Transgene today announced that new data confirm the ability of this novel investigational therapeutic cancer vaccine to induce immune responses against human papillomavirus (HPV) 16 antigens that are associated with antitumor response.
TG4001 is an investigational viral vector-based therapeutic cancer vaccine.
It is being evaluated in a randomized controlled Phase II clinical study comparing TG4001 with avelumab to avelumab alone in patients with HPV16-positive anogenital tumors.
The data presented on June 5, 2023, were generated from 46 patients in both trial arms.
- TG4001 induced the priming of adaptive immunity
- 58% of patients receiving TG4001 + avelumab showed an increase of immune responses against HPV antigens versus 9% in the avelumab arm. At baseline, immune responses against HPV antigens were limited to 4/46 patients.
- An immune response was detected at day 43 and tended to increase intensity at day 85.
- These data demonstrate that Transgene's TG4001 could induce a specific immune response against the antigens vectorized within this vaccine.
- 11 of the 13 patients with an immune response had either stable disease, partial or complete tumor response according to RECIST criteria.
- Remarkably, two case studies are presented, with patients exhibiting a strong E6 and E7 immune response while showing a complete clinical response.
- Transgene anticipates that the last patient will be randomized in the current Phase II clinical study in the first half of 2024. The final results will be communicated in 2024.
Dr. Alessandro Riva, MD, Chairman and CEO of Transgene, commented in a press release, "These data further confirm that our therapeutic vaccine TG4001 can induce clinically meaningful immune responses that are associated with antitumor response."
The abstract and poster can be accessed on the ASCO and Transgene websites.
TG4001 is an investigational therapeutic vaccine based on a non-propagative, highly attenuated Vaccinia vector, which is engineered to express HPV16 antigens (E6 & E7) and an adjuvant (IL-2).
TG4001 was designed to have a two-pronged antiviral approach: to alert the immune system specifically to cells presenting the HPV16 E6 and E7 antigens that can be found in HPV16-related tumors and to further stimulate the infection-clearing activity of the immune system through interleukin 2 (IL-2).
HPV is a group of more than 200 related viruses, some of which are spread through sex. Researchers previously confirmed that infection with HPV16 precedes the development of some head and neck cancers.
Other HPV cancer vaccine news is posted by Precision Vaccinations.

The journal Nature recently published a study that concluded "something happened" in mid-2021, making avian influenza (bird flu) Highly Pathogenic Avian Influenza viruses much more infectious.
While emphasizing that the risk to humans remains low, the experts who spoke to AFP Paris on June 3, 2023, indicated bird flu reports this year were a cause for concern, reported France24.
Published on May 29, 2023, a new study found the spread of A(H5N1) viruses worldwide, and detecting infections in mammals (foxes, skunks, bobcats, mountain lions, bears) and humans in the United States is noteworthy.
Except for pockets of endemic clade activity in South and Southeast Asia, clade 2.3.4.4b viruses have predominated over other A(H5Nx) clades over the past 18 months. As a result, clade 2.3.4.4b viruses have become entrenched in Asia, Europe, and probably in parts of Africa.
The increasing prevalence of 2.3.4.4b viruses are also changing the disease dynamics in Europe, with the potential for a transition from epizootic to enzootic status.
Our data highlight how quickly things can change in a natural system, and the potential for further A(H5Nx) reassortment and phenotypic diversification will only increase as the unprecedented global distribution of these viruses broadens, wrote these researchers.
As of May 2023, the Pan American Health Organization reported clade 2.3.4.4b had been detected in 16 countries in Latin America, the Caribbean, the United States, and Canada.
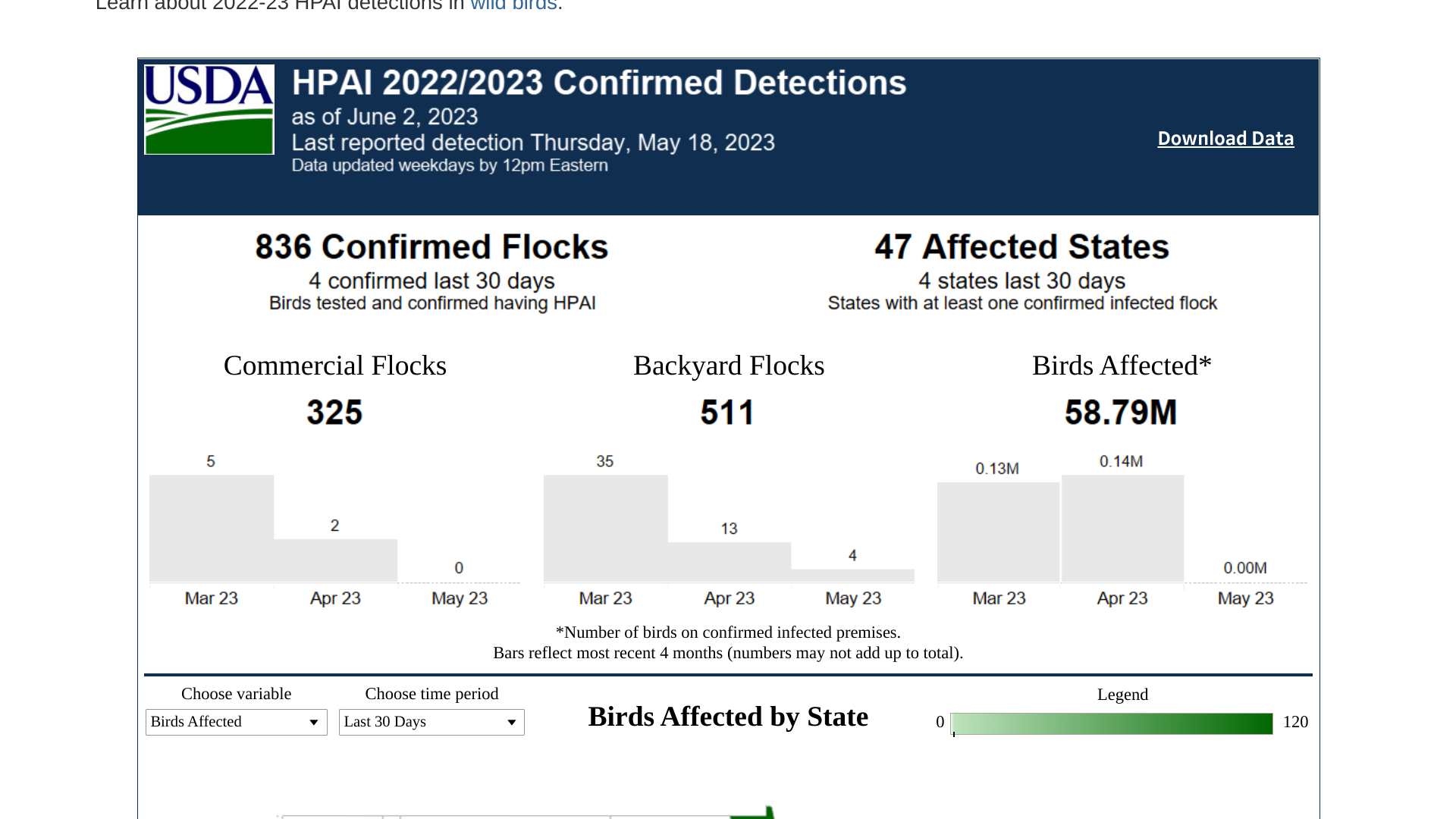
In the U.S., over 58 million birds in 47 states have been impacted since early 2022.
In the case of a bird flu pandemic in 2023, the U.S. government has already approved vaccines and continues investing in newer technologies. Furthermore, annual flu shots are unlikely to protect people during avian influenza (bird flu) pandemics.
As of June 4, 2023, bird flu outbreak news is posted at Precision Vaccinations.
When it comes to immunotherapy, a tumor’s classification as hot or cold is essential. Hot refers to tumors showing signs of inflammation, meaning the immune cells have recognized and responded to the malignant cells, wrote Kim Polacek, APR, CPRC, on June 4, 2023.
These tumors respond well to immunotherapy because the immune system is already activated.
Cold tumors, on the other hand, contain immune suppressive cells and are more challenging to treat.
Breast cancer was once regarded as a cold tumor disease and therefore difficult to treat with immunotherapy, but that is changing thanks to research happening at Moffitt Cancer Center.
A new clinical trial led by Dr. Heather Han is evaluating a combination therapy utilizing two immunotherapies (dendritic cell vaccine and pepinemab), along with trastuzumab, followed by adoptive T cell therapy, for patients with metastatic HER2 positive breast cancer.
“The goal of this therapy is to activate the immune system and create a hot tumor environment so we can collect those immune cells to create a specific cellular immunotherapy to better target their cancer,” said Dr. Han, clinical research director in the Department of Breast Oncology at Moffitt.
Information about this phase 1 clinical trial is being presented at the American Society of Clinical Oncology Annual Meeting. The clinical trial is ongoing. Han says this combination therapy could provide a new option for HER2-positive breast cancer patients with metastatic disease.

The U.S. Food and Drug Administration (CDC) confirmed on June 2, 2023, that the agency had revoked the emergency use authorization (EUA) of the Janssen COVID-19 Vaccine known as Jcovden®.
On May 22, 2023, Janssen Biotech Inc. requested the voluntary withdrawal of the EUA for this vaccine.
Previously, Janssen informed the FDA that the last lots of the vaccine purchased by the U.S. Government had expired, there is no demand for new lots of the vaccine in the U.S., and they do not intend to update the strain composition of this vaccine to address emerging variants.
On February 27, 2021, Johnson & Johnson announced that the FDA issued an EUA for this recombinant, replication-incompetent adenovirus, non-mRNA COVID-19 vaccine.
As of June 4, 2023, the authorization or approval status of the Jcovden COVID-19 vaccine varies by country.

The U.S. Department of State updated its Travel Advisory for the Republic of Peru regarding civil unrest.
On June 1, 2023, the State Department's Level 2: Exercise Increased Caution advisory confirmed visitors should not travel to:
- The Colombian-Peruvian border area in the Loreto Region.
- The Valley of the Apurímac, Ene, and Mantaro Rivers, including areas within the Departments of Ayacucho, Cusco, Huancavelica, and Junin.
- The Puno Region, including the Peruvian side of Lake Titicaca, and the Apurimac Region.
And the U.S. Embassy in Lima recently reported a 24-hour strike is expected to affect the Puno region on May 31, 2023. In addition, roadblocks could disrupt travel within the area and the city of Puno.
Furthermore, U.S. travelers participating in Ayahuasca and Kambo ceremonies should be aware that numerous persons, including U.S. citizens, have reported that while under the influence of these substances, they have witnessed or been victims of sexual assault, rape, theft, serious health problems and injuries, and even death.
Previously, Machu Picchu reopened to visitors in late February 2023 after a short closure.
If you visit Peru, the State Department suggests enrolling in the Smart Traveler Enrollment Program to receive alerts in an emergency.
From a health perspective, the U.S. CDC included Peru in its recent dengue outbreak advisory.
A new repository of drug-dependence technical reports and resources was launched by the World Health Organization (WHO) on June 1, 2023, empowering its audience to source information within a single webpage.
The repository from WHO’s Expert Committee on Drug Dependence (ECDD) is an essential resource for health professionals, drug policy experts, and policy-makers, as many of the substances reviewed by ECDD have otherwise limited information regarding their public health risk.
It represents the only online, freely accessible collection of information and reports on new psychoactive substances and medicines for medical and scientific use, comprising over 450 substances.

The U.S. Centers for Disease Control and Prevention (CDC) is issuing this Health Alert Network (HAN) Health Update CDCHAN-00492 to supplement the HAN issued on May 17, 2023, offering updates on the ongoing fungal meningitis outbreak.
As of June 1, 2023, a multistate outbreak of fungal meningitis is ongoing among patients who underwent procedures under epidural anesthesia in the city of Matamoros, Tamaulipas, Mexico, at two clinics: River Side Surgical Center and Clinica K-3.
A total of 212 residents in 25 U.S. states and jurisdictions have been identified who might be at risk of fungal meningitis because they received epidural anesthesia at the clinics of interest in 2023.
Three of these patients (two probable cases and one confirmed case) have died.
Various laboratories have detected fungal signals consistent with the Fusarium solani species complex from the cerebrospinal fluid (CSF) of patients receiving follow-up care in Mexico or the U.S.
In addition, elevated levels of beta-D-glucan, a biomarker of fungal infection, have been detected in the CSF of at least six patients. Efforts by public health officials are ongoing to find and notify additional patients who might be at risk.
Healthcare providers, public health officials, and the public should be aware that all patients, including those without symptoms, who underwent medical or surgical procedures under epidural anesthesia in Matamoros, Mexico, since January 1, 2023, should be evaluated for fungal meningitis as soon as possible.
As of June 2, 2023, the WHO advises against the application of any travel or trade restrictions on Mexico and the U.S. based on the current information available on this event.