Search API
WestVac Biopharma and West China Medical Center, Sichuan University, today announced Coviccine® Trivalent XBB.1.5-Recombinant COVID-19 Trivalent (XBB.1.5+BA.5+Delta) Protein Vaccine (Sf9 cell) was authorized by relevant authorities in China.
As of June 8, 2023, this is the first COVID-19 vaccine in the world authorized against XBB descendent lineages of the beta coronavirus SARS-CoV-2.
WestVac leveraged the rapid response of the insect cell expression platform in constructing the vector for Coviccine, which is of high purity and high quality for human use.
The subunit vaccine antigen is designed based on the structure of the targeting S-RBD and HR proteins of the COVID-19 subvariants XBB.1.5 and BA.5 and self-assembled into stable trimeric protein particles with squalene-based oil-in-water emulsion adjuvant added after purification and mixing.
This novel adjuvant significantly raises the titer of neutralizing antibodies, enabling the trimeric protein vaccine to induce a higher T-cell immune response.
Recent studies have shown that Coviccine induced a high titer of neutralizing antibodies against multiple subvariants.
Previously, the WHO Technical Advisory Group on COVID-19 Vaccine Composition advised on May 18, 2023, that new formulations of COVID-19 vaccines should aim to induce antibody responses that neutralize XBB descendent lineages.
As of the end of May 2023, the XBB family of variants accounted for about 90% of infections in China.
And in the U.S., over 90% of all variants are from the XBB family.

CNBC reported today the U.S. FDA's Antimicrobial Drugs Advisory Committee voted 21-0 to recommend full approval for Beyfortus® (Nirsevimab) for infants and young children.
The FDA considers committee recommendations when finalizing its decisions.
Beyfortu has already been approved in Canada, Europe, and the United Kingdom.
Beyfortus is the first extended half-life monoclonal antibody offering passive immunization to prevent lower respiratory tract infections caused by the respiratory syncytial virus (RSV).
AstraZeneca and Sanofi developed this RSV therapy.
This antibody therapy is not an RSV vaccine. The FDA recently approved two RSV vaccines for seniors.

The U.S. CDC's Morbidity and Mortality Weekly Report published today indicates about 66% of mpox vaccine–eligible persons remained unvaccinated.
From May 2022–April 2023, a total of 748,329 first JYNNEOS® (MVA-BN) vaccine doses (of the two recommended) were administered in the United States.
During the initial months of the outbreak, lower vaccination coverage rates among racial and ethnic minority groups were reported.
Focusing on the racial and ethnic groups with more significant mpox vaccination shortfalls, prioritizing resources, and improving access to vaccination for these groups can reduce the overall shortfall in mpox vaccination while promoting health equity.
However, after the implementation of initiatives developed to expand access to mpox vaccination, coverage among racial and ethnic minority groups increased, stated the CDC on June 9, 2023.

The World Health Organization (WHO) today reported over 10,000 fatalities related to COVID-19 were reported in the last 28 days, a decrease of 47% compared to April 10 to May 7, 2023.
During this 28-day reporting period, 144 of 243 (59%) countries and territories reported at least one COVID-19 case.
The situation at the regional level shows decreases in COVID-19 cases and fatalities in all six WHO regions.
Recently, the WHO and other authorities have announced their recommendations for updated COVID-19 vaccines for late 2023.
And the Forest plots displaying the effectiveness of COVID-19 vaccines against the Omicron variants are available on View-hub.org.
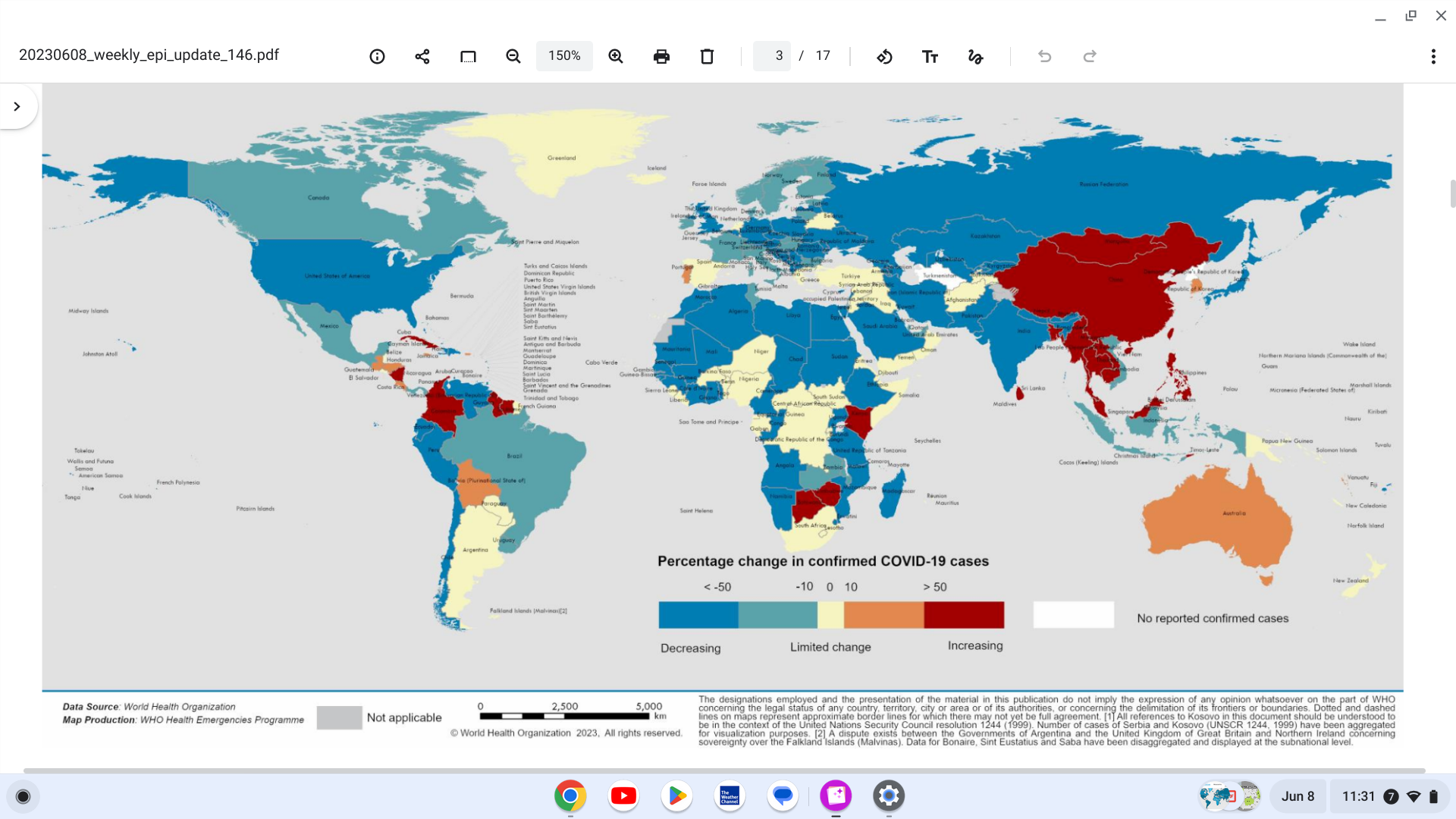
The outbreak of Marburg Virus Disease in Equatorial Guinea ended today with no new cases reported over the past 42 days after the last patient was discharged from treatment.
The outbreak, declared on February 13, was the first in Equatorial Guinea.
A total of 17 laboratory-confirmed cases and 12 deaths were recorded.
Five districts in four of Equatorial Guinea’s eight provinces were affected.
The western Litoral province Bata district was worst-hit, with 11 laboratory-confirmed cases reported. Among the reported cases, many were closely linked either through social gatherings and networks or geographically.
“While outbreak-prone diseases continue to pose a major health threat in Africa, we can bank on the region’s growing expertise in health emergency response to act quickly and decisively to safeguard the health and avert widespread loss of life,” said Dr. Matshidiso Moeti, World Health Organization (WHO) Regional Director for Africa, in a press release on June 8, 2023.
“The hard work by Equatorial Guinea’s health workers and support by partner organizations has been crucial in ending this outbreak. WHO continues to work with countries to improve measures to detect and respond effectively to disease outbreaks,” Dr Moeti added.
To support Equatorial Guinea’s response to the just-ended outbreak, WHO deployed experts in epidemiology, clinical management, health operations, logistics, risk communications, and infection prevention and control.
The Organization worked with the health authorities to set up a treatment center, provided medical supplies, including antivirals, and trained health workers in the critical aspects of outbreak control.
The WHO also supported the efforts by the authorities in neighboring Cameroon and Gabon to ramp up outbreak readiness and response.
Although the outbreak has ended, WHO continues to work with Equatorial Guinea to maintain measures such as surveillance and testing to enable prompt action should flare-ups of the virus occur, with the training provided during the outbreak helping to strengthen readiness capacity.
Marburg is in the same family as the virus that causes Ebola Virus Disease.
The Marburg virus is transmitted to people from fruit bats and spreads among humans through direct contact with the bodily fluids of infected people, surfaces, and materials.
In Africa, the first outbreak of Marburg was recorded in South Africa in 1975, followed by two others in Kenya in the 1980s. Since then, outbreaks have been reported in Angola, the Democratic Republic of the Congo, Ghana, Guinea, Uganda, and most recently, Equatorial Guinea and Tanzania.
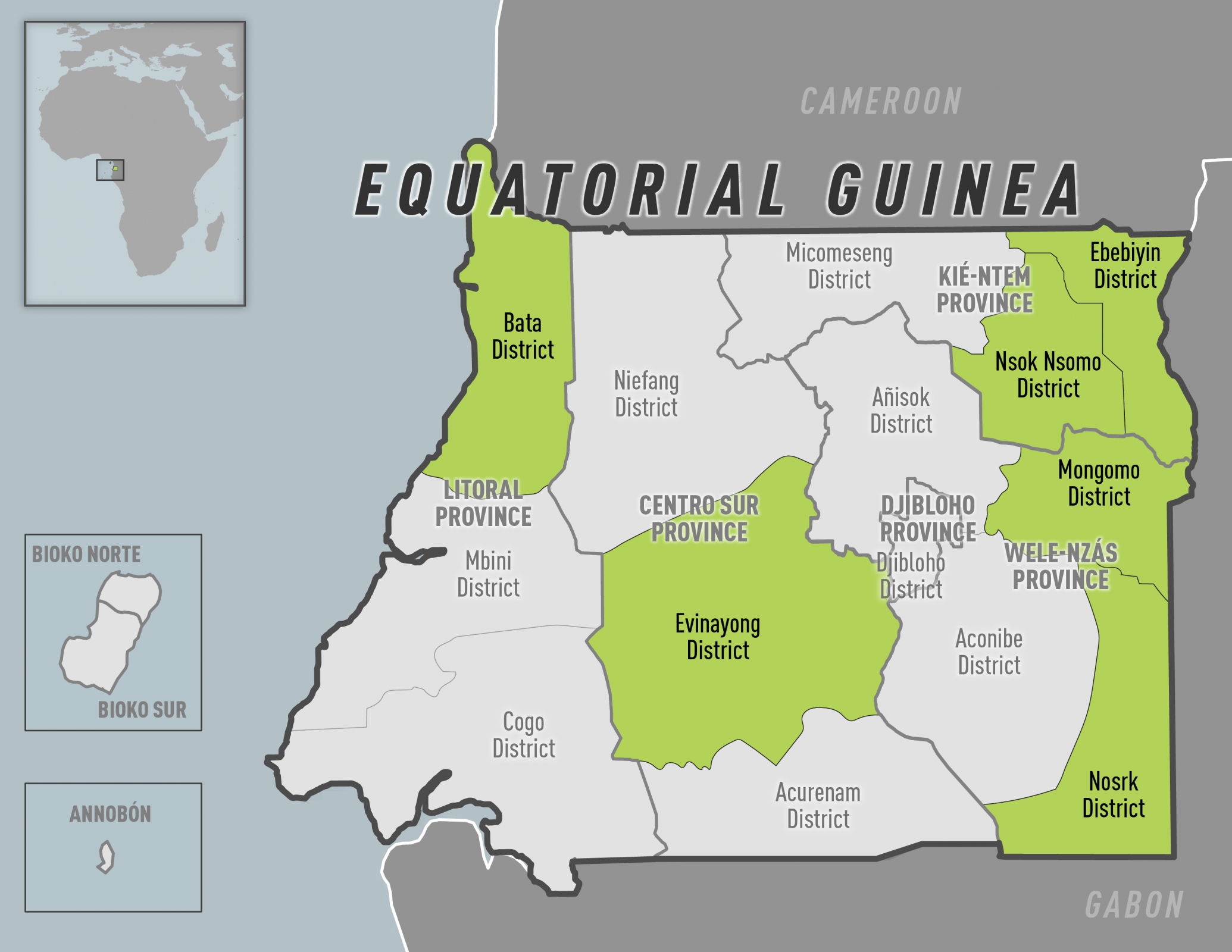
The U.S. Department of State today reissued its Level 2, Exercise Increased Caution travel alert for the Kingdom of Denmark.
This alert stated civil unrest events may occur at tourist locations, transportation hubs, markets/shopping malls, local government facilities, hotels, clubs, restaurants, places of worship, parks, major sporting and cultural events, educational institutions, airports, and other public areas at any time.
Furthermore, the U.S. Mission in Copenhagen issued a Demonstration Alert on June 7, 2023, reminding U.S. citizens to remain vigilant if in the area of demonstrations as these events can be unpredictable and quickly turn violent.
If you decide to travel to the Kingdom of Denmark, enroll in the Smart Traveler Program to receive local Alerts in an emergency.
From a health perspective, the U.S. CDC recently suggested prospective visitors confirm their immunization status with a healthcare provider before visiting Demark.
The USDA Animal and Plant Health Inspection Service (APHIS) recently announced it added Gabon, Guinea, and Moldova to the list of regions considered to be affected by highly pathogenic avian influenza (HPAI), known as bird flu.
Bird flu outbreaks are mainly located in areas of the Pacific Flyway.
The HAPI updates were confirmed on May 23, 2023.
On May 23, 2022, after confirming that the HPAI occurred in commercial birds or poultry, APHIS added Gabon to the regions where HPAI exists.
On June 3, 2022, the veterinary authorities of Guinea reported to WOAH an HPAI occurrence in that country.
On January 24, 2022, the veterinary authorities of Moldova reported to WOAH an HPAI occurrence in that country.
In the U.S., the Department of Agriculture's Animal and Plant Health Inspection Service reported the Eurasian (H5 clade 2.3.4.4b.) appeared in North America in January 2022 and has impacted over 58 million birds in 47 states as of June 2023.
Bird flu infections have also been detected in various mammals, such as bears, cats, dogs, dolphins, ferrets, foxes, minks, sea lions, and skunks.
As of June 7, 2023, bird flu vaccines are available for people, and vaccine candidates are being tested for birds.

The European Centre for Disease Prevention and Control (ECDC) and the European Medicines Agency (EMA) today issued a joint statement on adapted COVID-19 vaccines and considerations for their use during the upcoming 2023 vaccination campaigns.
In line with the outcome of meetings of international regulators and the World Health Organization (WHO) in May 2023, the EMA’s Emergency Task Force recommended on June 6, 2023, updating COVID-19 vaccines to target XBB strains (a subgroup of the SARS-CoV-2 Omicron strain), which have become dominant in Europe.
The EMA and ECDC also noted that monovalent vaccines (targeting only one strain) are a reasonable choice to protect people against dominant and emerging coronavirus strains.
Other COVID-19 vaccine news is posted by Precision Vaccinations.


