Search API
GSK plc today announced new data from the AReSVi-006 phase III trial evaluating the efficacy of a single dose of AREXVY™ against lower respiratory tract disease caused by the respiratory syncytial virus (RSV) in adults aged 60 years and older over multiple RSV seasons and after annual revaccination.
The results presented on June 21, 2023, show that one dose of AREXVY is efficacious against RSV-LRTD and severe LRTD over two full RSV seasons.
In a press release, Tony Wood, Chief Scientific Officer of GSK, stated, "These data show the efficacy of a single dose of our vaccine over two RSV seasons against RSV-LRTD, including in the populations most at risk due to age or underlying medical conditions."
"This reinforces our confidence in its potential to make a significant public health impact."
"We look forward to discussing these results with regulators and vaccine recommending bodies and collecting more data from the ongoing clinical development program."
As of June 2023, there are two approved RSV vaccines for older people in the U.S.
UPDATE - The ACIP voted to recommend AREXVY on June 21, 2023.

Brazil recently notified the World Health Organization (WHO) of a fatal laboratory-confirmed human case of infection with a swine-origin influenza A(H1N1) variant (v) virus in the state of Paraná.
The patient was a 42-year-old woman with underlying medical conditions who lived near a swine (pig) farm.
She developed a fever, headache, sore throat, and abdominal pain on May 1, 2023, and was hospitalized with a severe acute respiratory infection.
On May 4, the patient was admitted to the Intensive Care Unit, and she passed away on May 5, 2023.
Ongoing investigations reported that the patient did not have any direct contact with pigs, however, two of her close contacts worked at the swine farm.
The two contacts did not develop respiratory disease and tested negative for influenza.
To date, no human-to-human transmission associated with this case has been identified in Brazil.
This is the first human infection caused by an influenza A(H1N1)v virus reported in 2023 in Brazil and the third human infection reported in the state of Parana (2021 and 2022).
WHO assesses the risk of international disease spread through humans and/or community-level spread among humans posed by this event as low. The risk level will be amended if warranted by the investigations currently being conducted by national authorities.
As of June 20, 2023, there is no swine vaccine for Influenza A(H1N1)v infection currently licensed for use in humans.
And seasonal flu shots are generally not expected to protect people from influenza viruses that normally circulate in pigs.

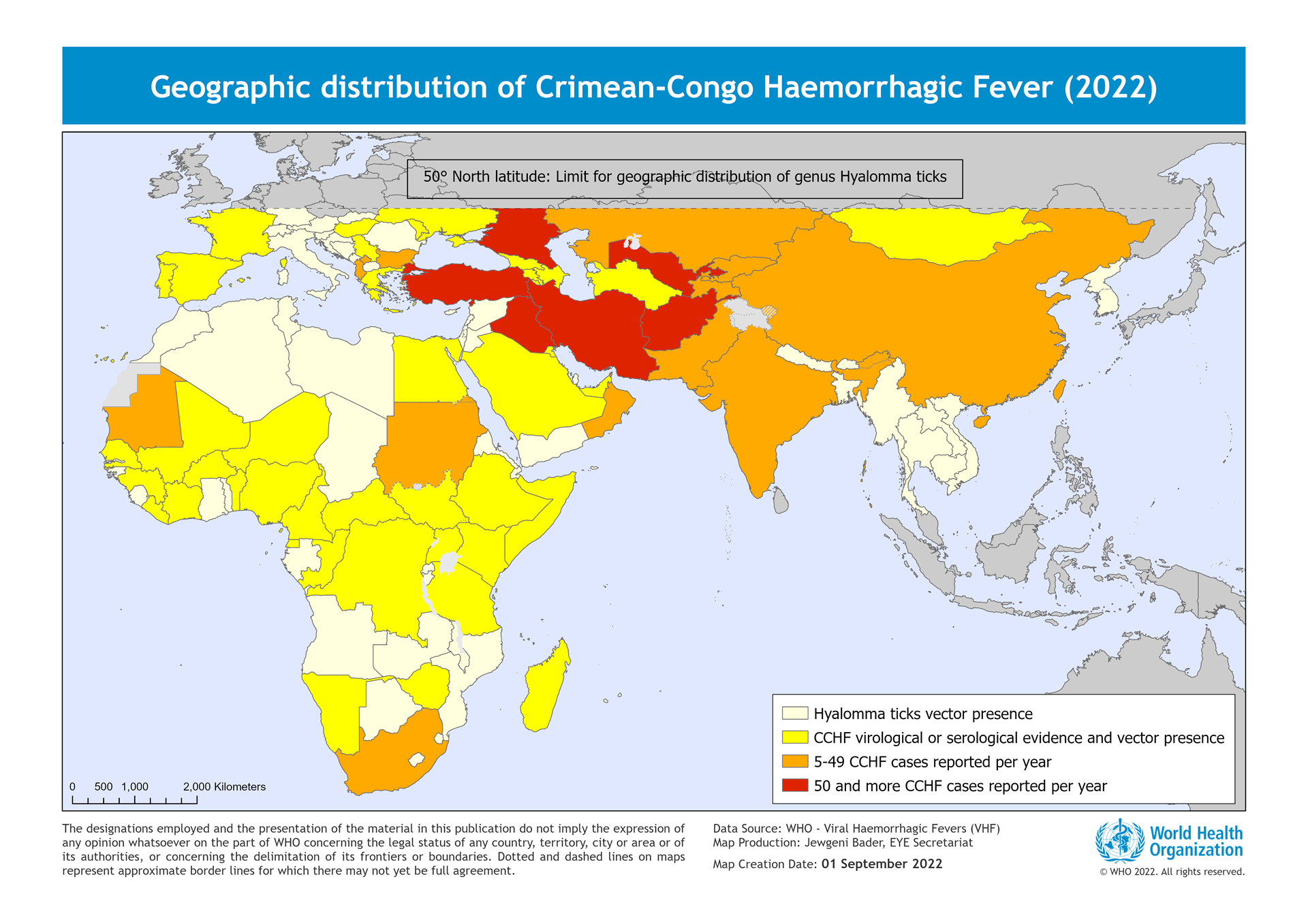
News18 recently reported the U.K.'s Science, Innovation, and Technology Committee was informed the Crimean-Congo hemorrhagic fever (CCHF) is highly likely heading in its direction, thanks to climate change.
We don't know what will arrive until it does, was a comment shared during the meeting on June 14, 2023.
Last year, the U.K. Health Security Agency confirmed a case of CCHF in England in a woman who had recently traveled to Central Asia. This was only the third case of CCHF imported to the U.K.
Outbreaks have been confirmed in Iraq, Namibia, and Pakistani in 2023. In Iraq, there were 219 confirmed cases of CCHF from January 2022 to late June 2022.
First described in the Crimean Peninsula in 1944, CCHF is endemic in all of Africa, the Balkans, the Middle East, and Asia, according to the World Health Organization (WHO).
The WHO says CCHF is a viral disease spread via ticks with a fatality rate of between 10 and 40%.
Human-to-human transmission of CCHF has been reported following close contact with blood, secretions, or other bodily fluids of infected persons. And cases have been reported among health workers caring for infected people.
Regarding preventive vaccines, the WHO published an overview of CCHF vaccine candidates.
And in March 2023, eBioMedicine published a study that supported further development of the ChAd platform expressing the CCHFV GPC to seek an effective vaccine against CCHFV.
In the U.K., no licensed human vaccine or approved medication targeting CCHF is available as of June 20, 2023.
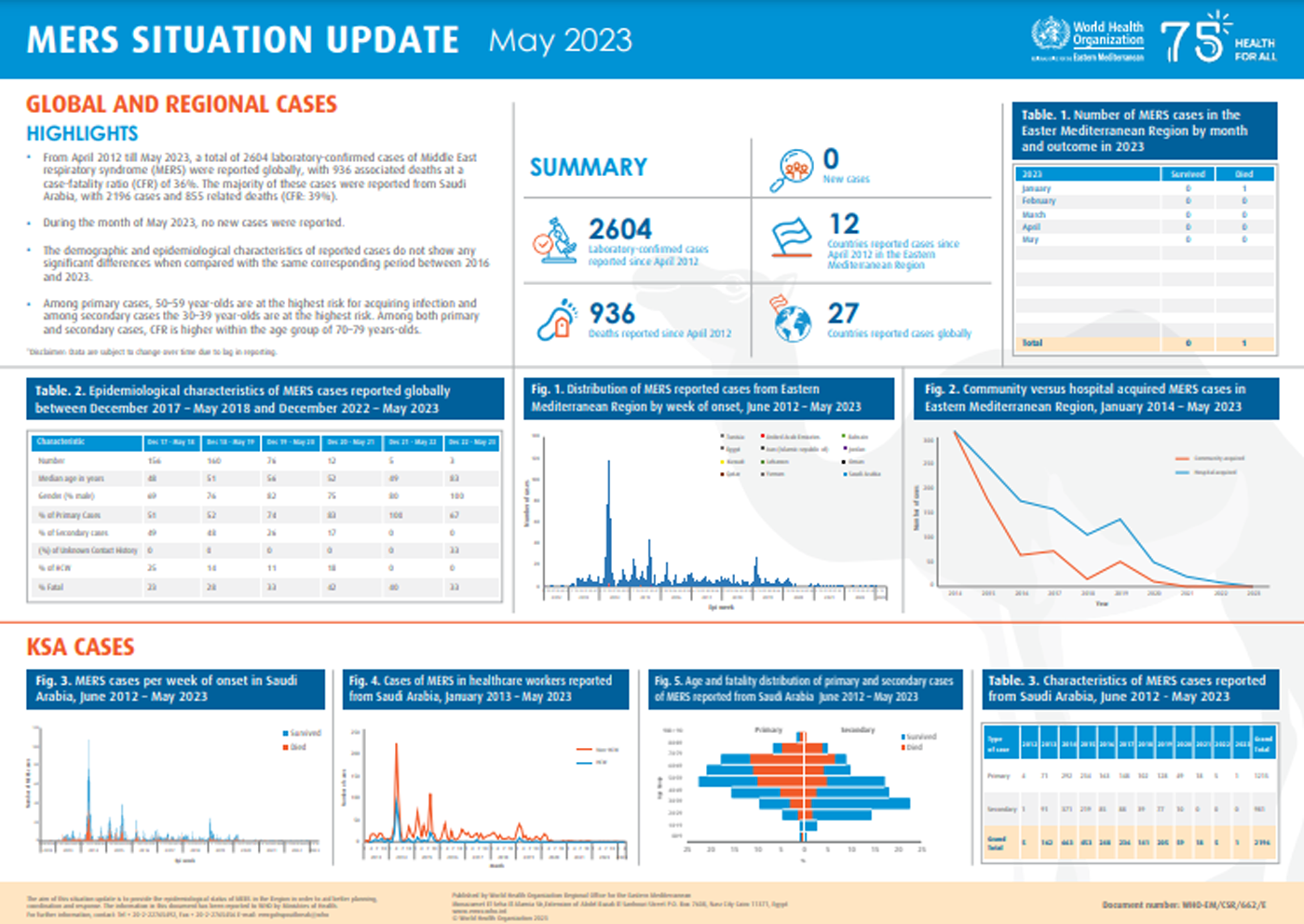
The World Health Organization (WHO) recently reported that from April 2012 until May 2023, 2,604 laboratory-confirmed cases of Middle East respiratory syndrome (MERS) were reported globally.
During May 2023, no new cases were reported.
As of June 20, 2023, there have been 936 associated deaths at a case-fatality ratio (CFR) of 36%.
Most of these cases were reported from the Kingdom of Saudi Arabia, with 2,196 cases and 855 related deaths (CFR: 39%).
Among primary cases, 50–59 year-olds are at the highest risk for acquiring a MERS infection; among secondary cases, 30–39 year-olds are at the highest risk.
Among both primary and secondary cases, CFR is higher within the age group of 70–79.
As of June 2023, there are no MERS vaccines authorized.
The U.S. CDC's Advisory Committee on Immunization Practices (ACIP) is scheduled to conduct an in-person and digital meeting beginning on June 21, 2023.
This three-day ACIP meeting will debate and vote on various vaccine recommendations, such as Respiratory Syncytial Virus vaccines for older adults.
Please see the agenda for the time and day.
Anyone can attend the digital meeting for free to listen and learn how the U.S. government makes vaccine recommendations.
The ACIP comprises medical and public health experts who develop recommendations on the use of vaccines in the civilian population of the U.S. The recommendations are public health guidance for safe use of vaccines and related biological products.
The Committee's recommendations are forwarded to CDC's Director for approval. Once approved, they are published in CDC's Morbidity and Mortality Weekly Report.
And ACIP's recommendations result in the official U.S. adult and childhood immunization schedules.

Bavarian Nordic A/S today announced today the initial safety and immunogenicity results from a randomized, double-blind, placebo-controlled Phase 3 clinical trial of a virus-like particle (VLP)-based chikungunya virus (CHIKV) vaccine candidate CHIKV VLP (PXVX0317) in healthy adults.
The initial results up to Day 22 post-vaccination showed that CHIKV VLP was immunogenic in healthy adults ≥65 years of age, as demonstrated by a strong induction of CHIKV neutralizing antibodies in 87% of vaccinees with neutralizing antibody titres exceeding the threshold agreed with authorities as a marker of seroprotection, thus meeting the primary endpoints of the study.
Importantly, seroprotective neutralizing antibodies were also observed in most subjects (82%) at Day 15 post the single vaccination, demonstrating a fast onset of protection for the VLP-based CHIKV vaccine candidate.
This clinical trial will continue for a 6-month follow-up for both safety and immunogenicity.
Paul Chaplin, President and CEO of Bavarian Nordic, said in a press release on June 20, 2023, “While we still await the results from the second and larger Phase 3 study in adolescents and adults later this year, these highly encouraging results provide a high degree of confidence for our CHIKV vaccine program.”
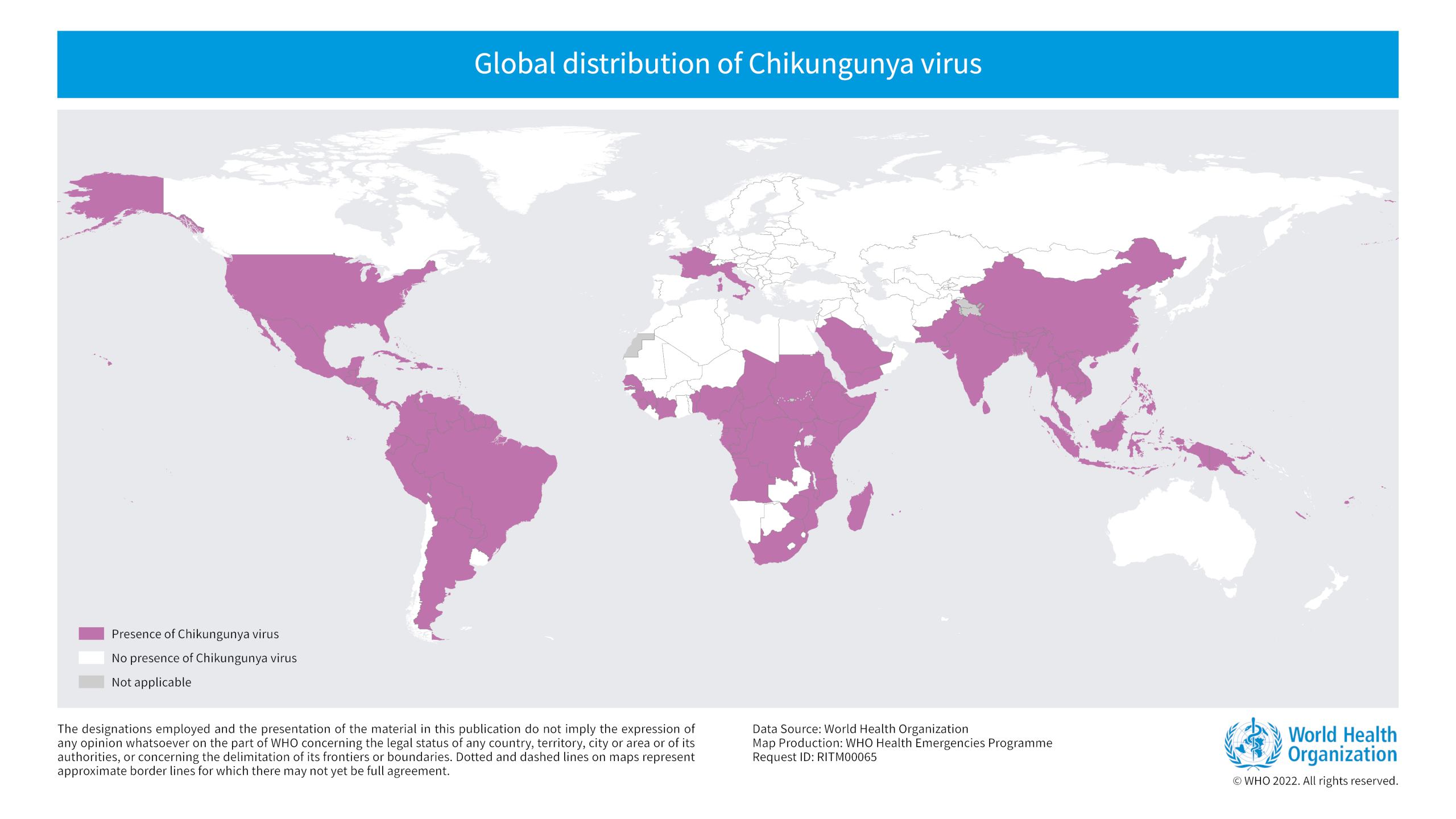
Chikungunya is a mosquito-borne viral disease caused by the Chikungunya virus (CHIKV), causing outbreaks in over 100 countries as of 2023.
From 2006–2021, 4,590 chikungunya cases in U.S. travelers were reported to the U.S. CDC.
While mortality is low, morbidity is high; nearly 50% of individuals with CHIKV disease have debilitating long-term symptoms that can intensify with age.
Additional Chikungunya vaccine news is posted by Precision Vaccinations.
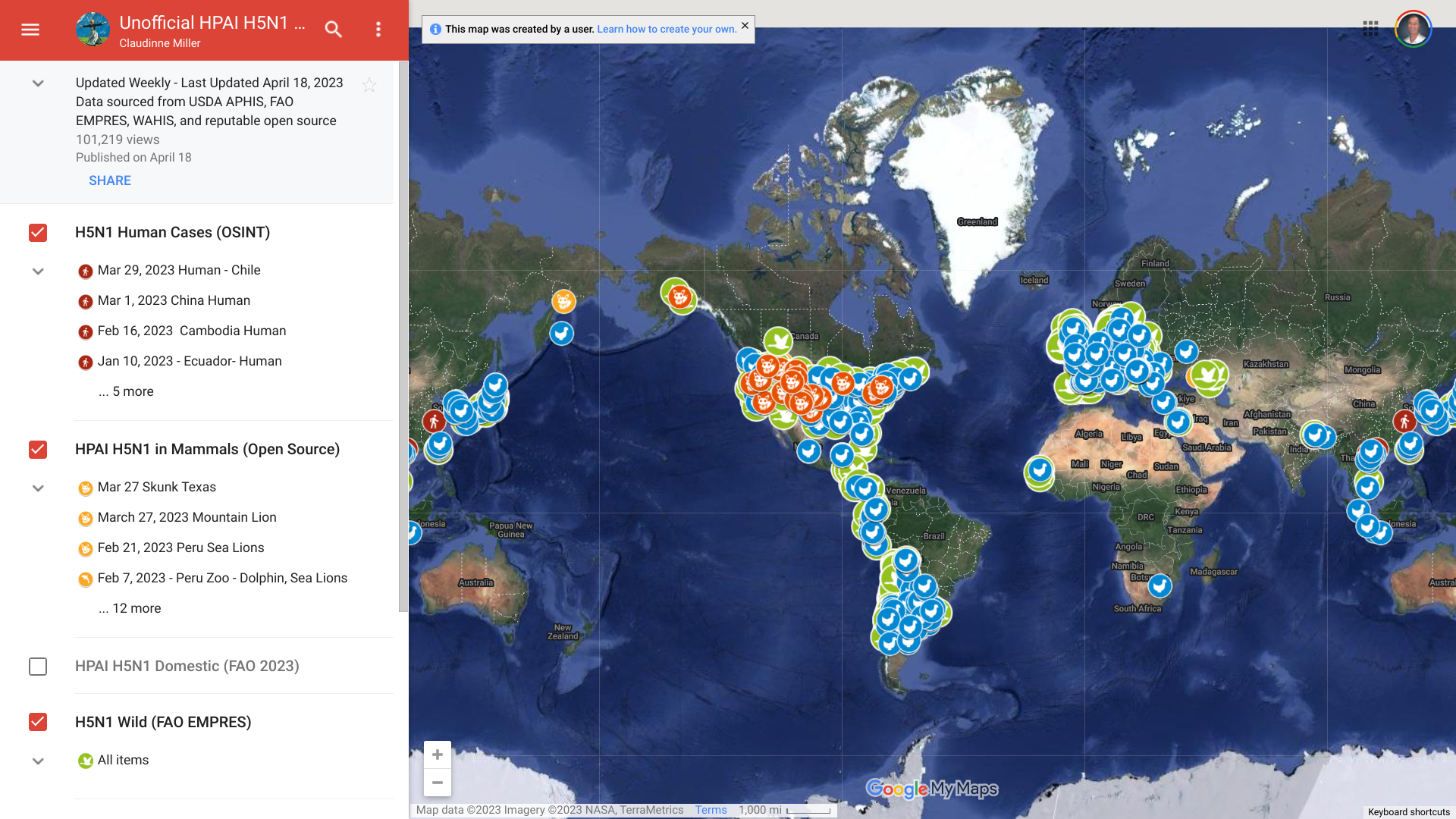
The European Centre for Disease Prevention and Control (ECDC) recently confirmed sporadic human cases of avian influenza A (H9N2) have been observed in 2023, mainly in young children.
As of June 12, 2023, one new human infection with H9N2 was reported in Sichuan province, China.
And since 1998, a total of 125 laboratory-confirmed cases, including two deaths, of human infection with H9N2 viruses have been reported in eight countries: China (112), Egypt (4), Bangladesh (3), Cambodia (2), Oman (1), Pakistan (1), India (1), and Senegal (1).
Over the past year, various humans have been infected with different avian influenza viruses.
According to the Centers for Disease Control and Prevention, Avian influenza or bird flu is caused by infection with avian (bird) influenza (flu) Type A viruses.
And the Highly Pathogenic Avian Influenza (HPAI) strain of the H5N avian flu is currently spreading in the U.S.
In the U.S., the government has already authorized one bird-flu vaccine and invested in developing other vaccine candidates.
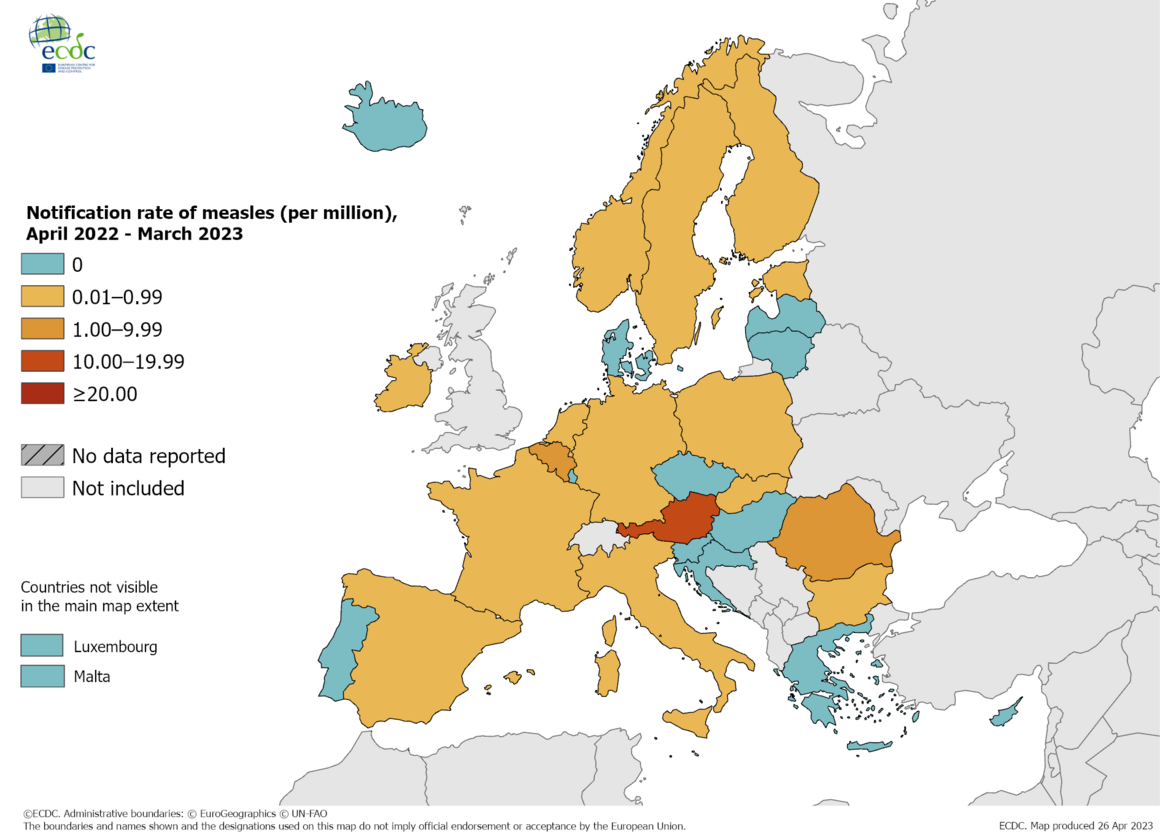
The European Centre for Disease Prevention and Control (ECDC) recently announced that since the beginning of 2023, about 242 measles cases have been reported by 12 European countries.
Austria reported 121 cases of measles in 2023.
According to national data, as of June 13, 2023, Styria is the most affected region in Austria, with 102 cases reported since the beginning of the outbreak in early 2023.
Measles outbreaks have also been reported from other regions, including Upper Austria (5), Lower Austria (4), Vienna (5), Carinthia (4), and Burgenland (1).
As of June 14, 2023, surveillance sources had detected 22 new suspected and/or confirmed measles cases reported in seven EU/EEA countries over the past month: Estonia (2), Germany (12), Ireland (2), Poland (2), Spain (1), and Sweden (1).
So far, in 2023, one measles-related death has been reported in the EU/EEA (the Netherlands).
And the U.S. CDC reported on June 12, 2023, India has confirmed 73,536 measles cases over the past year.
In the U.S., a total of 16 measles cases were reported by 11 jurisdictions as of early June 2023.
Measles is a very contagious disease that is preventable with various vaccines.