Search API
Sorrento Therapeutics, Inc. today announced the full enrollment in a pivotal Phase 3 study of the oral Mpro inhibitor, Ovydso (STI-1558), in mild or moderate symptomatic adults infected with SARS-CoV-2, or COVID-19.
Sorrento anticipates that top-line data from the study will be available in the third quarter of 2023.
Once the data is finalized, Sorrento plans to open discussions with regulatory authorities worldwide to discuss the path required for each particular authority for full approval of Ovydso.
If the trial meets its endpoints, the company has agreements with the China Health Authority and the National Medical Products Administration (NMPA) for an application review.
“We are pleased to see that Ovydso has enrolled quickly for successful completion of enrollment for the phase 3 pivotal trial in China. We look forward to seeing the final data and to working closely with the NMPA during the review to evaluate this as a potential stand-alone treatment for COVID-19 patients as rapidly as possible,” stated Henry Ji, Ph.D., Chairman and CEO of Sorrento, in a press release on June 26, 2023.
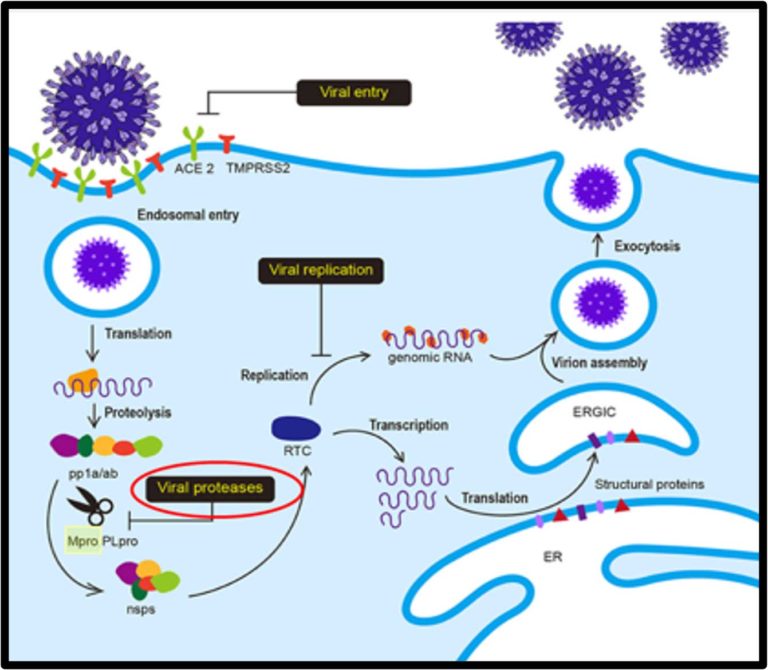
STI-1558 is a prodrug, and its active form AC1115 binds to Cys-145 of the catalytic domain of Mpro, which is 100% conserved in all SARS-CoV-2 variants and achieves a broad-spectrum anti-SARS-CoV-2 activity.
STI-1558 is also a Cathepsin L inhibitor, which may block effective viral entry into host cells without accelerating viral mutations.
Sorrento is a clinical and commercial stage biopharmaceutical company based in California.
Pfizer Inc. recently announced that the U.S. Centers for Disease Control and Prevention’s (CDC) Advisory Committee on Immunization Practices (ACIP) unanimously voted to recommend PREVNAR 20® (20-valent Pneumococcal Conjugate Vaccine) for routine use to help protect infants and children from invasive pneumococcal disease (IPD) caused by the 20 Streptococcus pneumoniae serotypes covered by the vaccine and for the prevention of otitis media in infants six weeks through five years of age caused by the original seven serotypes contained in PREVNAR®.
These provisional recommendations will be reviewed and finalized by the director of the CDC and the Department of Health and Human Services.
“We are thrilled with today’s ACIP decision as it recognizes the increased level of protection that PREVNAR 20 will provide to millions of infants and children against pneumococcal disease,” said Luis Jodar, Ph.D., Chief Medical Affairs Officer, Vaccines/Antivirals and Evidence Generation, Pfizer, in a press release on June 22, 2023.
Previously, the European Medicines Agency approved the brand name Apexxnar in February 2022.

The Florida Health Department recently published an updated Mosquito-Borne Disease Surveillance that revealed additional dengue and malaria patients in Florida.
As of June 17, 2023, the Weekly Arbovirus Report confirmed:
- Four cases of dengue were reported in persons with international travel. In 2023, 88 travel-associated dengue cases were reported. And two cases of locally acquired dengue have been reported.
- Another case of locally acquired malaria was reported in Sarasota County. In 2023, two cases of locally acquired malaria have been reported.
In response to these mosquito-transmitted diseases, Florida has issued alerts for Sarasota and Manatee counties regarding malaria.
And in Miami-Dade County, a dengue alert was issued in April 2023.
The Florida Department of Health is working with local partners and county mosquito control in these areas to mitigate the risk of further mosquito transmission.
Florida continues statewide surveillance for mosquito-borne illnesses, including West Nile virus, Eastern equine encephalitis, St. Louis encephalitis, malaria, chikungunya, and dengue.
From a vaccination perspective, one dengue vaccine is approved in the U.S., and malaria vaccines are currently available in Africa as of June 23, 2023.

The Florida Department of Health (DOH) in Sarasota and Manatee Counties recently confirmed a second locally-acquired malaria case.
As of June 19, 2023, this patient was being treated for this mosquito-cause infection.
This case has been identified as the P. vivax malaria species, which is not as fatal as other species.
In response, a mosquito-borne illness alert was issued for Sarasota and Manatee counties.
On May 26, 2023, DOH released information on the first confirmed local malaria case, who was treated and recovered.
Effective treatment is readily available through Florida hospitals and other healthcare providers.
The Florida Department of Health confirmed aerial and ground mosquito spraying is being conducted in these counties to mitigate the risk of further transmission.
Malaria is not transmitted from person to person.
Only infected Anopheles mosquitoes can transmit malaria to humans.
Malaria infects approximately 219 million people each year, with an estimated 660,000 deaths, mostly children in Africa.
The U.S. Centers for Disease Control and Prevention (CDC) recently stated the risk of locally acquired malaria in the U.S. is extremely low.
About 2,000 malaria cases are diagnosed annually in the U.S., most in international travelers.
However, Florida has had malaria outbreaks in the past.
In 2003, eight locally acquired P. vivax malaria cases were reported in Palm Beach County, FL.
From a prevention perspective, malaria vaccines have already been approved.
The World Health Organization recommended the Mosquirix™ malaria vaccine in 2021, and the R21/Matrix-M™ vaccine was approved for use in Africa in 2023.

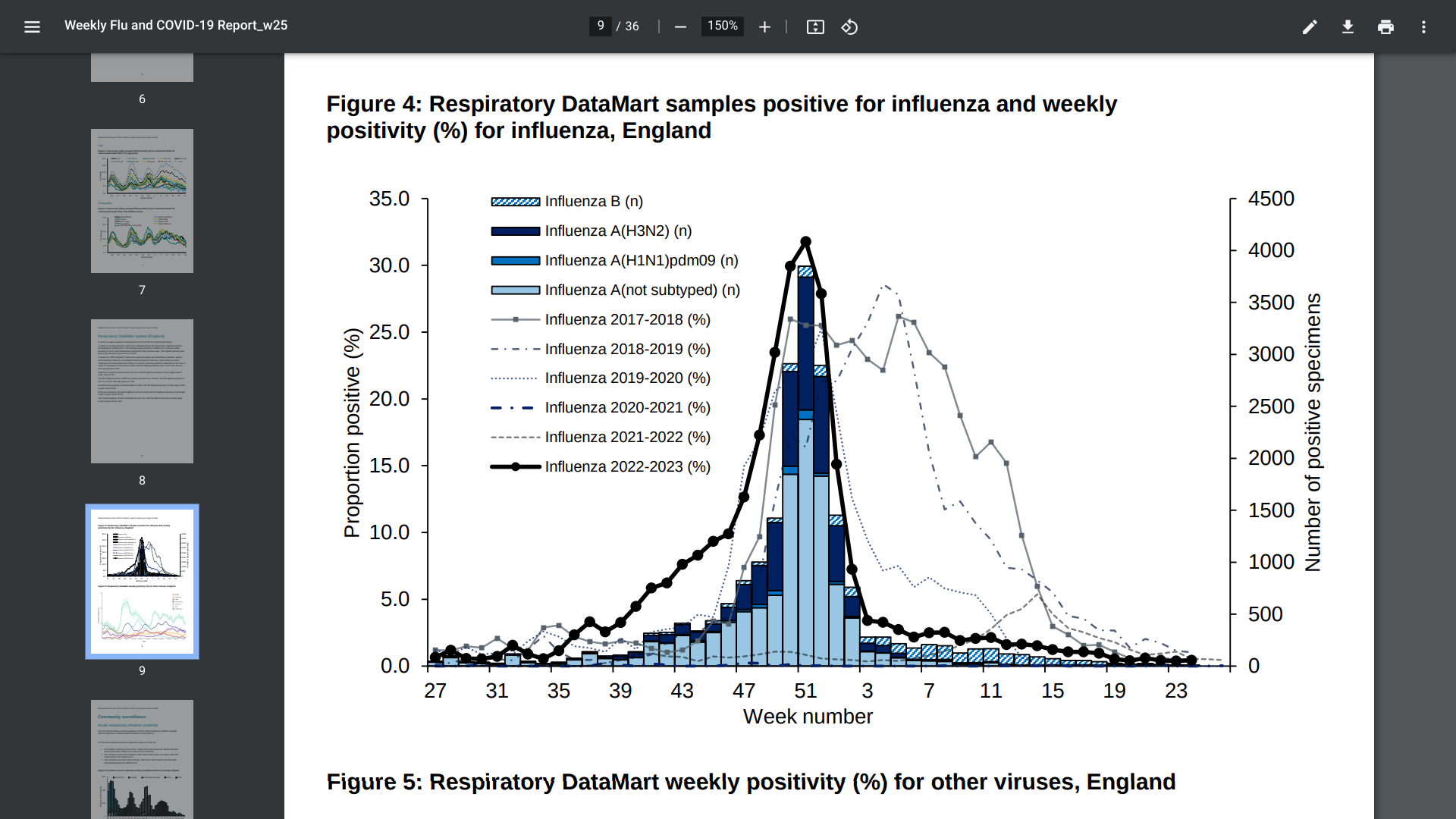
The UK Health Security Agency (UKHSA) recently reported that during week #24, from most indicators, influenza activity remained stable in the United Kingdom.
As of June 22, 2023, the UKHSA confirmed there were no influenza confirmed outbreaks were reported in England.
In week #24, influenza remained low and stable at 0.4% compared with the previous week, with the highest positivity seen in the 15 to 44 age group at 0.9%.
And influenza ICU admissions remained low and stable within the baseline range of activity.
Other flu seasonality news is posted by Precision Vaccinations.
Invivyd, Inc. recently announced positive initial data from its ongoing Phase 1 clinical trial of its lead investigational monoclonal antibody (mAb) candidate, VYD222.
VYD222 is a broadly neutralizing, half-life extended mAb candidate in development for the prevention of symptomatic COVID-19 in vulnerable populations, such as immunocompromised people.
Initial Phase 1 data show that a single administration of VYD222 was generally well-tolerated at all three dose levels tested, with no serious adverse events reported to date.
At the lowest VYD222 dose tested (1500 mg), geometric mean serum neutralizing titers were 3245.1 (95% CI: 1882.5, 5594.0) against Omicron XBB.1.5 at Day 7, with a geometric mean 38.87-fold rise (95% CI: 10.3, 146.8) from baseline to Day 7 (n=8).
Greater VYD222 dose levels are designed to provide greater protection from any potential loss of neutralization activity as SARS-CoV-2 evolves.
Dave Hering, chief executive officer of Invivyd, stated in a press release on June 22, 2023, "Based on previously published clinical data from randomized controlled clinical trials, we believe that mAb directed against the receptor binding domain of the SARS-CoV-2 spike protein offer an attractive safety profile, even at higher doses, and that strong serum neutralization activity would be predictive of clinical benefit."
Additional COVID-19 monoclonal antibody news is posted by Precision Vaccinations.

The U.S. Centers for Disease Control and Prevention (CDC) today announced the mpox outbreak in Chicago, Illinois, has reached 40 individuals.
As of June 23, 2023, the CDC's Morbidity and Mortality Weekly Report confirmed that mpox vaccine breakthrough cases were reported to the Chicago Department of Public Health.
The observed proportion of cases among persons who had received JYNNEOS® (MVA-BN) smallpox/mpox or ACAM2000 smallpox vaccines in this cluster was unusual.
Mpox breakthrough cases averaged eight months since the last vaccination.
During March 18–June 12, 2023, 40 laboratory-confirmed mpox cases were identified in Chicago, including 22 (55%), five (13%), and 13 (33%), respectively, among patients who had received two doses of JYNNEOS or one dose of ACAM2000 vaccine, those who had received one vaccine dose of JYNNEOS vaccine, and those who had not received any vaccines for mpox.
All cases occurred among persons assigned male sex at birth; 37 (93%) identified as male and 28 (70%) as gay.
The median age was 33 (IQR = 23–49).
Eleven (28%) patients were living with HIV, 10 of whom had received two doses of JYNNEOS or one dose of ACAM2000 vaccine and whose HIV was well-controlled (CD4 count >200 cells/mm3 and viral load <200 viral copies/mL).
Three (8%) patients experienced concurrent sexually transmitted infections during mpox diagnosis.
Of six live births, two neonates developed lesions within one week after their mothers became symptomatic.
This investigation is ongoing. However, no similar clusters are being seen elsewhere in the U.S.
Preliminary sequencing indicates the virus is the same B.1 variant of Clade IIB, the predominant variant of the 2022–2023 outbreak.
The CDC wrote that this increase in mpox cases before significant summer events scheduled nationwide and in Chicago raised concerns about possible future case increases.
Although the cause of this cluster has not yet been determined, leading hypotheses include a potentially high number of sexual exposures in a network with many vaccinated persons, decreased vaccine effectiveness due to waning of humoral immunity, or vaccine mishandling or administration errors.
Mpox outbreak news and other sexually transmitted disease vaccine news are posted by Precision Vaccinations.