Search API
PharmaJet® recently announced the implementation of a study in Nigeria to evaluate the impact of intradermal vaccine administration of fractional inactivated poliovirus vaccine (fIPV) using their Tropis® ID Needle-free Injection System (NFIS).
Announced on October 24, 2023, the study, in collaboration with the National Primary Health Care Development Agency, Jhpiego, PATH, and the Sydani Group, is assessing coverage rates and potential cost reductions associated with using Tropis for fIPV delivery as compared to the current standard of intramuscular delivery of full dose IPV using needle and syringe.
Partners are also evaluating the acceptability and feasibility of using needle-free from the healthcare worker and caregiver perspective.
The study will run through January 2024, with children at 22 urban and rural health facilities receiving fIPV with Tropis. Evidence from the study is intended to inform policy regarding intradermal delivery of polio vaccine in routine immunization settings.
Paul LaBarre, Vice President of Global Business Development, PharmaJet, commented in a press release, “This (effort) builds on our experience in Pakistan where Tropis has been demonstrated to increase coverage rates. Including additional campaigns in Somalia, we have provided more than 10 million syringes for polio immunization programs."
"On this World Polio Day 2023, we restate our commitment to the Global Polio Eradication Initiative.”
As of October 30, 2023, the U.S. CDC confirms over thirty countries have reported polio outbreaks in the past year.
To mark World Polio Day on October 24, 2023, supporters from over 30 countries joined the Make Polio History campaign to tell governments that eradication is possible and urgently needed.
According to the U.S. Centers for Disease Control and Prevention (CDC), there are thirty-one countries reporting polio cases in 2023.
The CDC says polio is a crippling and potentially deadly disease that affects the nervous system. Some people have only minor symptoms, such as fever, tiredness, nausea, headache, nasal congestion, sore throat, cough, stiffness in the neck and back, and pain in the arms and legs.
In rare cases, polio infection causes permanent loss of muscle function (paralysis). Polio can be fatal if the muscles used for breathing are paralyzed or if there is an infection of the brain.
Countries are deploying an innovative, novel oral polio vaccine type 2 (nOPV2) to better address the evolving risk of type 2 circulating vaccine-derived poliovirus (cVDPV2).
The vaccine is a modified version of the type 2 monovalent OPV vaccine, which clinical trials have shown provides comparable protection against poliovirus while being more genetically stable and less likely to be associated with the emergence of cVDPV2 in low immunity settings.
Thus, nOPV2 can be a significant tool for more sustainably stopping polio outbreaks.
As of October 29, 2023, approximately 820 million doses of nOPV2 had been administered across 35 countries. An additional 16 countries have met the requirements for using nOPV2 in the event of a polio outbreak.
However, the nOPV2 is not authorized by the U.S. Food and Drug Administration.
Since 2000, the only polio vaccine used in the U.S. is the inactivated polio vaccine (IPV), which protects against severe disease, including paralysis. IPVs are generally available at clinics and pharmacies in the U.S.
Tuberculosis (TB) is not only the world's biggest infectious killer, but it also destroys families and livelihoods. Besides the fear of dying from the disease, in many communities, a diagnosis can sentence someone to social isolation, wrote Linda Geddes in an article published by Gavi, the Vaccine Alliance, on October 24, 2023.
While the Bacillus Calmette–Guérin (BCG) vaccine provides significant protection against TB disease in infants and young children, new vaccines that block infection and prevent TB disease are urgently needed.
More than 100 years have passed since the first administration of the BCG vaccine; hopes are building that a vaccine that could protect all age groups against all types of TB may finally be in reach.
Fortunately, several TB vaccine candidates are now in late-stage clinical trials, raising hopes that an affordable and effective vaccine may soon be within reach.
Today's BCG vaccines are based on different attenuated strains of M. bovis.
These BCG vaccines are recommended for newborns in countries with a high burden of TB.
TB vaccines are among the most widely used, reaching more than 80% of infants in countries where the BCG vaccine is included in routine childhood immunisation programs.
In the United States, vaccinations with the TICE® BCG version are limited and generally offered to children in areas experiencing community spreading of TB.
The U.S. CDC reported in March 2023 that TB cases increased by 5% in 2022, with 8,300 confirmed cases. In addition, about 13 million people live with latent TB infection in the U.S.
Recent data indicates TB rates are accelerating by double digits in certain Texas areas (Dallas, Hidalgo County, Houston, San Antonio) in 2023.
Gedde's full, unedited article is posted at this link.

While the World Health Organization (WHO) Influenza Update N° 456 says respiratory syncytial virus (RSV) activity was generally low or decreasing globally, new data indicates the United States is seeing measurable increases.
The U.S. Centers for Disease Control and Prevention (CDC) RSV detection graphs showed increases throughout the U.S. as of October 27, 2023.
The National Respiratory and Enteric Virus Surveillance System (NREVSS) reports that the weekly percentage of RSV polymerase chain reaction (PCR) test positivity was 10.9% as of October 21, 2023.
During the 2022–2023 RSV season, positive test results peaked in November.
From an age perspective, the Weekly Emergency Department Visits chart clearly indicates that the most affected by this RSV season have been children under one-year-old.
In 2022, the JAMA Network conducted an Original Investigation that found 96 (95% CI, 92-99) RSV deaths among children younger than one year. And a study published by the Journal of Infectious Diseases determined that RSV-related deaths in infants <1 year peaked at about one month.
Furthermore, a meta-analysis of eleven studies published in October 2023 found that the meta-estimate of RSV-positive tests among pregnant women was 3.4% (95% CI: 1.9; 54).
According to the CDC, RSV has geographic trends in the U.S.
Florida's RSV season is longer than the rest of the country and has distinct regional patterns.
The Florida Department of Health reported as of week #42, October 21, 2023, RSV activity was increasing in hospital admissions and emergency room rates, with current outbreaks in Martin (1), Pinellas (2), and Volusia (1).
Fortunately, for the first time, there are approved RSV vaccines and passive immunizations available in the U.S. this season.

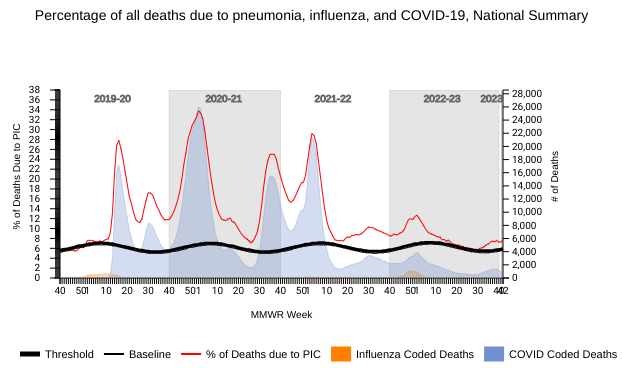
The U.S. National Center for Health Statistics (NCHS) Mortality Surveillance reported on October 27, 2023, that most respiratory disease deaths last week were related to pneumonia, not COVID-19 or influenza.
During week #42, the NCHS reported 1,379 pneumonia deaths, compared with 637 COVID-19 and 20 influenza.
While these diseases have U.S. FDA-approved vaccines available in 2023, preventing pneumonia deaths is more complicated, as viruses, bacteria, and fungi can all cause pneumonia.
According to the U.S. CDC, pneumonia is an infection of the lungs that can cause mild to severe illness in people of all ages.
Globally, pneumonia claimed the lives of 2.5 million people, including 672,000 children, in 2019 alone.
Vaccines can help prevent most pneumonia cases caused by pneumococcus bacteria, SARS-CoV-2, or influenza viruses.
These immunizations include
- COVID-19
- Haemophilus influenzae type b
- Influenza
- Measles
- Pertussis
- Pneumococcal
- Respiratory syncytial virus
- Varicella
According to the CDC, these immunizations are safe and may be coadministered. But side effects can occur which are usually mild and go away on their own within a few days.
These vaccines are generally available at health clinics and pharmacies in the U.S.
Note: The NCHS data presented on October 27, 2023, are preliminary and may change as more data are received and processed.
Moderna, Inc. recently announced that the first participant has been dosed in a Phase 3 study of the Company's combination vaccine candidate against influenza and COVID-19 (mRNA-1083) in the U.S.
As of October 24, 2023, the Phase 3 study is expected to enroll approximately 8,000 adults and will evaluate the immunogenicity, safety, and reactogenicity of mRNA‐1083 as compared with active control, co‐administered licensed influenza and SAR‐CoV‐2 vaccines in two independent age‐group sub‐study cohorts.
The mRNA-1083 candidate selected to advance to Phase 3 achieved hemagglutination inhibition antibody titers similar to or greater than those of both licensed quadrivalent influenza vaccines and SARS-CoV-2 neutralizing antibody titers similar to those of the Spikevax bivalent booster in the Phase 1/2 study.
mRNA‐1083 has the potential to efficiently reduce the overall burden of acute viral respiratory diseases by providing simultaneous protection against influenza and SARS‐CoV‐2 viruses in a single injection.
mRNA‐1083 offers greater convenience and has the potential to lead to increased compliance with vaccine recommendations.
This approach could benefit public health by synergistically increasing coverage rates against influenza and SARS‐CoV‐2 viruses.
The Company continues to target a potential initial regulatory approval for the combination vaccine in 2025.

Araclon Biotech recently announced encouraging final results from its Phase 2 clinical trial of ABvac40, an active vaccine against the Aβ40 peptide, for treating patients with early-stage Alzheimer's disease (AD).
The results show that ABvac40 had a favorable safety profile, elicited a robust immune response against Aβ40, and demonstrated some potential cognitive benefits in early-stage AD patients.
The vaccine candidate met the primary endpoints and showed differences between the vaccine- and placebo-treated groups in some secondary exploratory endpoints.
ABvac40 is uniquely designed to target the C-terminal end of the Aβ40 peptide.
Thus, it is believed to prevent harmful reactions and avoid immune triggers responsible for meningoencephalitis, a complication observed in earlier AD vaccines.
Emerging research suggests that Αβ40 plays a role in cerebral amyloid angiopathy, a highly prevalent condition among the growing number of AD patients.
Notably, although the clinical trial was not powered for finding efficacy on neuropsychological scales, the ABvac40-treated group exhibited as much as a 38% reduction in disease progression, as reflected by the Mini-Mental State Examination score, suggesting ABvac40's potential efficacy in addressing the cognitive decline associated with AD.
Other neuropsychological tests, such as the Repeatable Battery for the Assessment of Neuropsychological Status and the Trial Making Test, showed favorable results on ABvac40 compared to the placebo group.
Global or functional scales did not show differences between the ABvac40 and placebo groups. In addition, volumetric magnetic resonance imaging showed a lesser increase in whole-brain atrophy in the ABvac40 group compared to the placebo group.
"We are pleased to report final positive results from the Phase 2 study of ABvac40, including a robust immune response with some significant reduction in disease progression, all with a favorable safety profile," said Jose Terencio, Ph.D., Araclon chief executive officer and vice president of Grifols Innovation and New Technologies, in a press release on October 25, 2023.
"Previous vaccines in development for AD faced setbacks due to harmful meningoencephalitis side effects."
"The results reported for ABvac40 to date validate its clinical potential, positioning it as a promising therapeutic candidate for early AD treatment. We look forward to evaluating the next steps for this program."
As of October 27, 2023, the U.S. FDA has not approved a vaccine targeting AD.