Search API
GC Biopharma announced today that it has applied to the Korean Ministry of Food and Drug Safety for the marketing approval of "GC1109", an anthrax vaccine jointly developed by the company and the Korea Disease Control and Prevention Agency (KDCA).
If approved, "GC1109" will be the world's first recombinant anthrax vaccine.
'GC1109' contains a protective antigen as its active pharmaceutical ingredient, produced by recombinant DNA technology. This delivers two types of proteins, comprising anthrax toxin, lethal factor, and edema factor, into cells.
In the Phase II clinical trial, the vaccine's immunogenicity and safety were demonstrated with healthy volunteers. Subjects who received intramuscular administration of GC1109 generated antibodies sufficient to neutralize anthrax toxins, while adverse drug reactions or solicited adverse events were similar to those of the placebo group.
In the animal efficacy study, GC1109 induced neutralizing antibody, which remained at a high level even six months after the 4th dose of the vaccine, with a high survival rate against the bacillus anthracis spore challenge.
GC Biopharma stated in a press release on November 5, 2023, "We believe in the significance of our journey to localize the anthrax vaccine in terms of securing vaccine sovereignty while promoting public health and national security."
"GC Biopharma will continue to lead the localization of critical medicines and contribute to the stable supply of basic medical supplies as it has been doing for other vaccines and blood products since the foundation of the company."
Since it is unethical to expose human volunteers to lethal Bacillus anthracis, and field trials are not feasible due to a low incidence of anthrax, human clinical efficacy studies for an anthrax vaccine cannot be conducted.
In such cases, under the "Animal Rule" of The Special Act for Promotion of the Development and Emergency Supply of Medical Products in Response to Public Health Crisis, animal efficacy data can be used to establish the vaccine's clinical benefit if the evidence from the animal studies provides substantial grounds for the product's effectiveness.
Anthrax, caused by Bacillus anthracis, is a Class 1 infectious disease by the Infectious Disease Control and Prevention Act, with a lethality rate of up to 97% if untreated.
To prepare against potential bioterrorism and consequent national crisis, GC Biopharma, under the research project supported by KDCA, has been developing a recombinant vaccine for anthrax since 2002.
GC Biopharma (formerly Green Cross Corporation) is a biopharmaceutical company that delivers life-saving and life-sustaining protein therapeutics and vaccines.
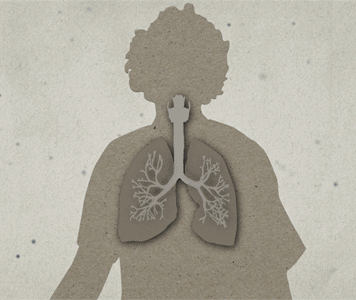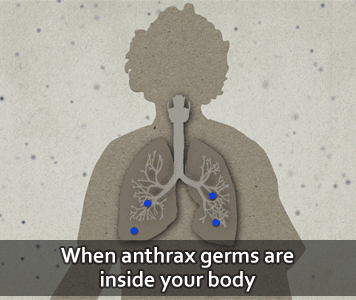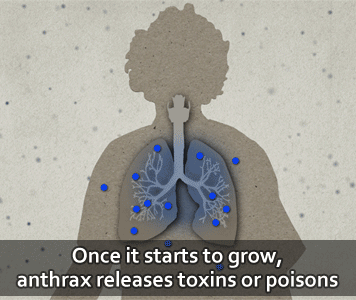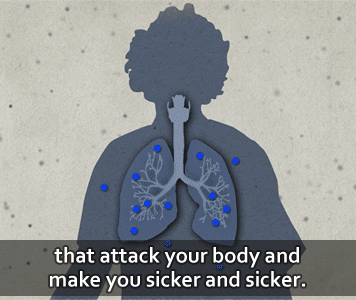
According to the U.S. CDC, people get infected with anthrax when spores get into the body. When this happens, the spores can be activated and become anthrax bacteria.
Then, the bacteria can multiply, spread out in the body, produce toxins (poisons), and cause severe illness.
The good news is anthrax is not contagious.
You cannot catch anthrax from another person the way you might catch a cold or the flu.
In rare cases, person-to-person transmission has been reported with cutaneous anthrax, where discharges from skin lesions might be infectious, says the CDC.
Moffitt Cancer Center today announced researchers are working to improve the efficacy of neoantigen-targeted cancer vaccines by better understanding whether primary or metastatic tumors should be used to produce the personalized vaccine.
On November 5, 2023, these cancer specialists launched a study evaluating primary and metastatic tumor pairs from 45 patients with several solid tumor types, including melanoma, bladder, head and neck cancers, and non-small cell lung cancer.
Results presented at the Society for Immunotherapy of Cancer annual meeting show that melanoma, bladder, and head and neck tumors share a high percentage of mutations between primary and metastatic tumors.
However, other solid tumors, such as esophageal and non-small cell lung cancer, share less.
Whole exome sequencing was used to identify somatic alterations, which are genetic mutations or DNA alterations that may impact the type of antigens produced by the cancer cells that the vaccine can then target.
Dr. Ahmad Tarhini, Director, Cutaneous Clinical and Translational Research at Moffitt, commented in a press release, "Our analysis demonstrates genetic variations that exist when comparing paired primary and metastatic tumors that appear to vary by histology."
"Variants are potentially undergoing negative selection supported by the preferential loss of out-of-frame events in metastatic tumors."
Understanding the clonal structure will be vital to predicting neoantigens for effective neoantigen-based vaccines, where oncogenic drivers can be prioritized and used to determine the primary clones.
Tarhini and the Moffitt team continue this work, expanding their study to include paired tumor samples from 600 additional patients.
As Florida's only National Cancer Institute-designated comprehensive cancer center and one of only 30 leading cancer centers in the U.S. participating in the National Comprehensive Cancer Network, Moffitt is at the forefront of cancer centers worldwide.

The U.S. Food and Drug Administration (FDA) MedWatch Safety Alert recently published an advisory on X informing healthcare providers who administer the Moderna COVID-19 vaccine (SpikeVax® XBB.1.5) (2023-2024 Formula) to individuals six months through 11 years of age to ensure that the correct volume of the vaccine (0.25 mL) is withdrawn from the vial and that the correct dose is administered to the vaccine recipient.
On November 1, 2023, the FDA confirmed that it had become aware that some healthcare providers may not recognize that the single-dose vial of Moderna COVID-19 Vaccine (2023-2024 Formula) for use in individuals six months through 11 years of age contains notably more than 0.25 mL of the vaccine.
Some healthcare providers may be withdrawing the entire contents of the vial to administer to an individual.
The Dosage and Administration section of the Fact Sheet for Healthcare Providers Administering Vaccine has been revised to clarify that 0.25 mL should be withdrawn from the vial, and the vial and any excess volume should be discarded.
If healthcare providers, parents, or caregivers have questions, they may contact the FDA's Center for Biologics Evaluation and Research at [email protected].
On November 2, 2023, Moderna, Inc. reported financial results and provided business updates for the third quarter of 2023, but these did not highlight this MedWatch advisory.
"Through this quarter, we demonstrated our ability to increase our share in the U.S. market (Spikevax's U.S. market share to date increased to 45% from 36% in 2022). We now expect this year's vaccination rate to be similar to last fall," commented Stéphane Bancel, Moderna's CEO, in a press release.
"In the third quarter, we significantly resized our manufacturing infrastructure to make our COVID-19 franchise profitable for 2024 and beyond.
The Company reported $1.8 billion in Spikevax® vaccine sales in the third quarter of 2023. This led to $3.9 billion in vaccine sales for the year through the third quarter.
Moderna believes that the U.S. market for fall 2023 will be at least 50 million doses, supporting total 2023 Spikevax sales of at least $6 billion.

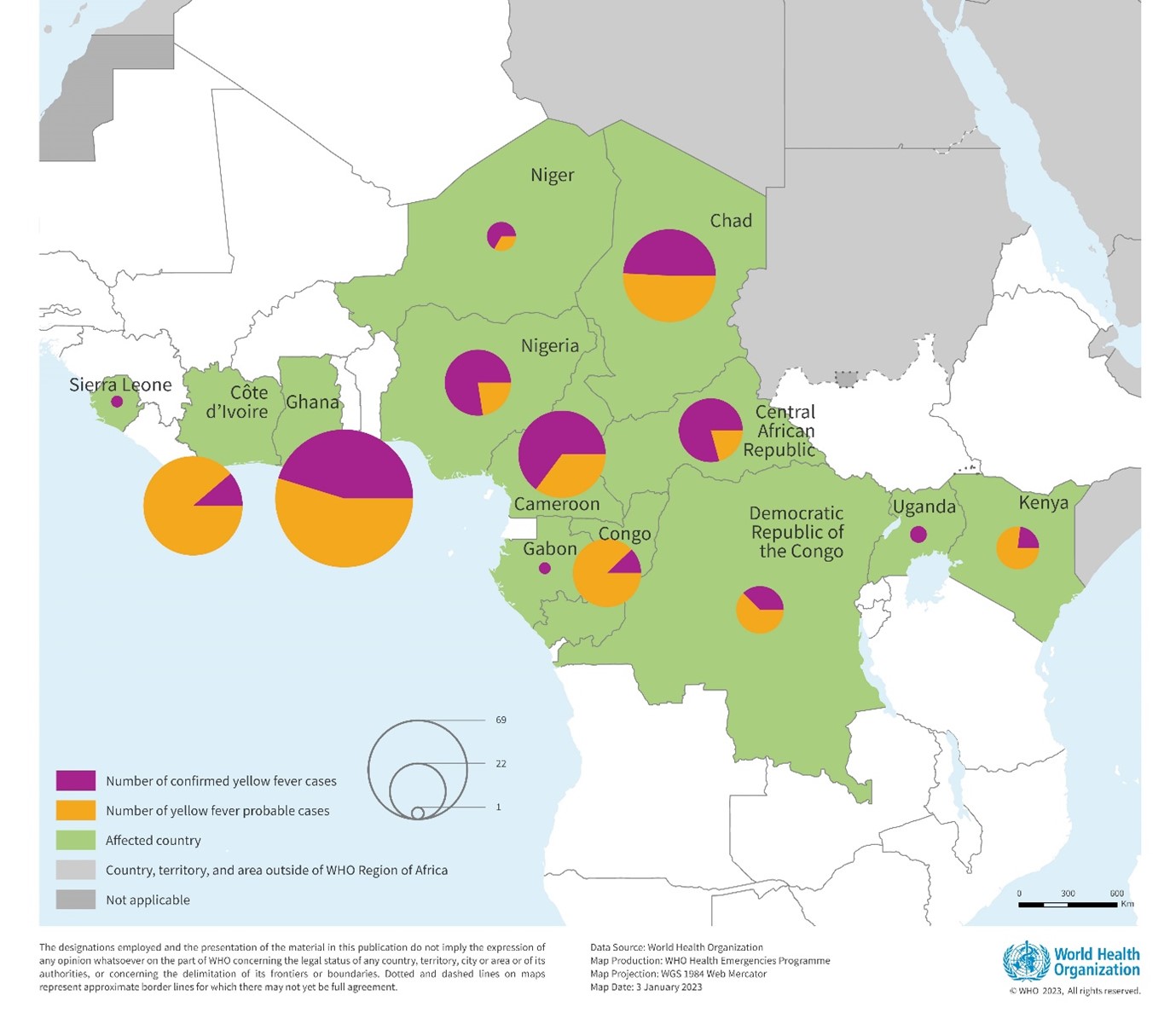
According to an update recently published by the Africa Centres for Disease Control and Prevention (Africa CDC), yellow fever outbreaks continue across the continent in 2023.
As of October 28, 2023, the Africa CDC reported a total of 2,779 yellow fever cases, and 36 deaths (CFR: 1.3%) have been reported in seven African Union countries this year.
The impacted countries are Cameroon (41 cases; 4 deaths), CAR (326; 5), Congo (324; 2), Gabon (79; 0), Guinea (178; 4), Nigeria (1,819; 21), and Uganda (12; 0).
In 2022, 12 countries in the African Region reported confirmed yellow fever cases.
Yellow fever is an epidemic-prone, vaccine-preventable disease transmitted to humans by mosquitoes. The incubation period ranges from 3 to 6 days. While many people do not experience symptoms, individuals can have more severe symptoms.
Death can occur within 7 - 10 days in about half of cases with severe symptoms.
According to the WHO/UNICEF Estimates of National Immunization Coverage in 2021, routine immunization coverage against yellow fever in the African Region for childhood vaccinations was 48%, much lower than the threshold required to confer population immunity.
In reaction to these data, the WHO and Africa CDC re-assessed the health risk at the regional level in 2022 as moderate.
Currently, the U.S. population is mostly unvaccinated against yellow fever, and the U.S. Strategic Stockpile has not secured vaccine reserves.
During a sizable epidemic in the U.S., the demand for yellow fever vaccines could surpass production capacities. Sanofi Pasteur's YF-VAX® vaccine is the only U.S. Food and Drug Administration-approved vaccine as of November 2023.
For many international travelers, proof of yellow fever vaccination is a requirement to visit at-risk countries, such as Brazil.
The Coalition for Epidemic Preparedness Innovations (CEPI) and the University of Oxford announced the launch of a new project to initiate early development of prototype vaccines against the Junín virus.
CEPI confirmed on October 2, 2023, that it would provide up to $25 million to Oxford for preclinical and Phase I clinical development of a vaccine against the Junín virus using Oxford's ChAdOx platform
This seldom-discussed virus was selected as an exemplar of the Arenavirus family, which includes the Lassa virus, Junin virus, Machupo virus, Guanarito virus, and lymphocytic choriomeningitis virus.
Arenavirus infections are responsible for multiple deadly hemorrhagic fevers with epidemic and pandemic potential. Junín virus can cause Argentine Haemmorhagic Fever, with symptoms including muscular pain, dizziness, rashes, and a 15-30% case fatality.
Dr. Richard Hatchett, CEO of CEPI, commented in a press release, "This new project will harness the University of Oxford's extensive vaccinology experience and its innovative ChAdOx vaccine technology – one of only a handful of vaccine platforms proven to work at speed, scale, and low cost – to expand the world's scientific knowledge on arenavirus vaccines."
"The project will generate vital resources for the proposed Global Vaccine Library, helping accelerate efforts to reduce vaccine development timelines to 100 days when faced with future threats."
The data and materials generated by this new project could give the world a head start in rapidly developing safe and effective vaccines against Arenaviruses within 100 days of their identification, potentially stopping a future pandemic in its tracks, wrote CEPI.

Vaxart, Inc. today announced it has dosed the first subject in its Phase 1 clinical trial evaluating Vaxart’s oral pill bivalent norovirus vaccine candidate (VXA-G1.1-NN) focused on lactating mothers.
This new study is evaluating the ability of oral vaccine tablets to induce breast milk antibodies and transfer antibodies to infants.
Norovirus sickens approximately 21 million people in the U.S. annually, including 15% of children under age 5. While pediatric deaths due to norovirus in the U.S. are rare, they are much more common in the developing world.
Currently, there are no U.S. FDA-approved norovirus vaccines.
The Phase 1, multicenter, randomized, double-blind, placebo-controlled, single-dose, dose-ranging VXA-NVV-108 Clinical Trial is designed to evaluate the safety, tolerability, and immunogenicity of orally administered bivalent GI.1/GII.4 norovirus vaccine in healthy lactating females at least 18 years of age and their breast-feeding infants (aged 30 days to 11 months).
The study is expected to enroll approximately 76 subjects at seven sites in South Africa.
“Initiating this study is an important step toward Vaxart’s goal of developing a vaccine that may reduce the significant global health threat norovirus poses to children under five years of age,” said Dr. James F. Cummings, Vaxart’s chief medical officer in a press release on November 2, 2023.
“We believe an oral norovirus vaccine pill may one day allow mothers to protect their infants against this highly contagious virus for which there currently is no approved vaccine.”
The Phase 2b study (VXA-NVV-201) is expected to add safety data that, if successful, could enable Vaxart to schedule an End-of-Phase 2 meeting with the U.S. FDA, potentially in 2024.
Vaxart believes that its proprietary pill vaccine delivery platform is suitable for delivering recombinant vaccines, positioning the company to develop oral versions of currently marketed vaccines and to design recombinant vaccines for new indications.

For the second time in a month, the mosquito-transmitted dengue fever virus has been confirmed in a person in Southern California.
On November 1, 2023, the City of Long Beach Department of Health and Human Services (Health Department) reported one case of dengue in a resident who has not traveled outside the U.S.
This is the first non-travel-related case of dengue in Long Beach, which has reported five travel-related cases.
Long Beach is home to approximately 466,000 Californians.
According to the Health Department, the risk of local exposure remains low.
"The health and well-being of the community is our most important priority," commented Long Beach Mayor Rex Richardson in a press release.
"We are working closely with health officials and doing everything we can to prevent more (dengue) cases. We ask that everyone do their part by removing standing water on their property to help us control the mosquitoes in our neighborhoods."
For more local information, people are encouraged to visit longbeach.gov/dengue.
According to reports, over 4.2 million infections and about 3,000 dengue outbreak-related deaths have been reported from 79 countries/territories in the past year.
In the U.S., the state of Florida has reported the most travel-related and local dengue cases in 2023.
An earlier locally-acquired dengue case was reported in Pasadena, California.
Dengue is a disease that is spread by the bites of Aedes species mosquitoes. When a mosquito bites someone with one of dengue's four viruses in their blood, it can spread the virus to others.
The best way to protect oneself from dengue and other diseases spread by mosquitoes is to avoid mosquito bites.
Dengue vaccines are available in 2023, but there are various geographic limitations and/or testing requirements.