Search API
A study led by Chicago Department of Public Health researchers published in the Journal of Infectious Diseases involved estimating rates of HIV, gonorrhea, and chlamydia among mpox patients.
This study was published on November 8, 2023, and identified factors related to mpox severity from June 2022 to March 2023.
These researchers concluded that sexually transmitted infections (STIs) could facilitate mpox transmission.
Of the 1,124 mpox patients, 44% had HIV, and 70% had a previous or current STI, with 39% having had at least three previous STI episodes.
Of 335 vaccinated mpox patients, 55% had received one dose of the JYNNEOS® (MVA-BN) vaccine, and 45% had received two doses.
In total, 17.6% has received one or more JYNNEOS vaccination before mpox infection.
The U.S. Centers for Disease Control and Prevention (CDC) reported on October 25, 2023, that post-JYNNEOS vaccination reinfection cases have been published and that they are aware of less than 10 cases of probable reinfection.
The CDC reported Vaccine Effectiveness of JYNNEOS against mpox ranges from 36%–75% for 1-dose vaccination and 66%–89% for 2-dose vaccination.
"Future research should examine predictors of mpox infection among those with STIs, including other STIs, such as syphilis, HIV risk at STI screening or anatomical site of infection," wrote these researchers.
As of October 2023, there has been a significant increase in mpox outbreaks in the European Region.
In the last month, 21 countries reported mpox cases, with Portugal reporting the highest relative increase in cases (n = 86).

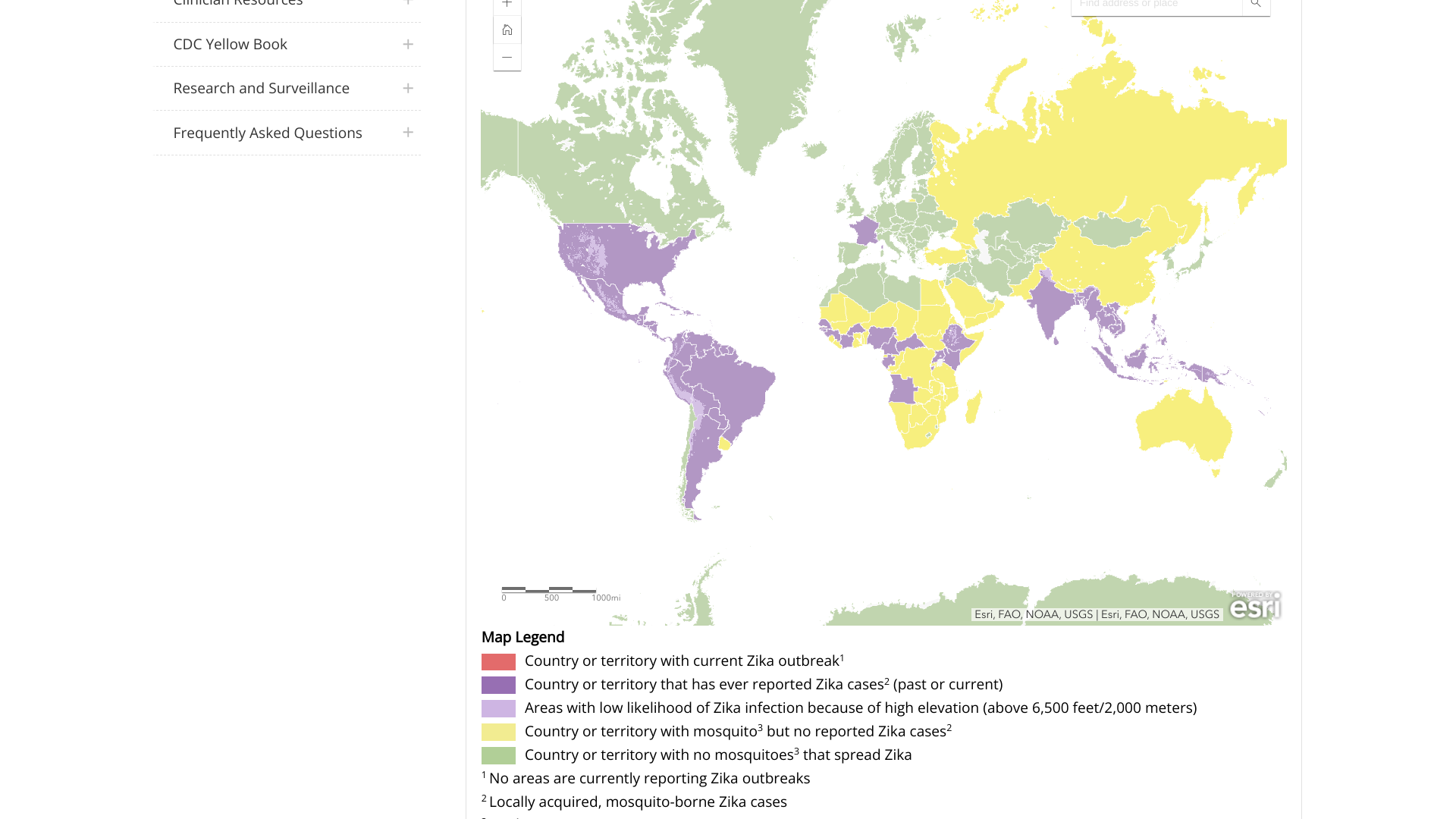
While most people assume Zika virus outbreaks ended a few years ago, new data from the Pan American Health Organization (PAHO) clearly indicates that Zika remains a significant health risk.
As of November 8, 2023, the PAHO dashboard reported 28,267 Zika cases from countries in the Americas, primarily in Central and South America.
The leading countries reporting Zika outbreaks are led by Brazil, with over 26,000 cases, followed by Bolivia, Belize, Columbia, Paraguay, and Venezuela.
In the United States, Puerto Rico's Weekly Report on Arboviral Diseases shows 33 probable Zika cases this year and 20 cases in 2022.
Zika is primarily spread to people by the bite of a mosquito infected with the virus, but it can also be passed during sex from a person infected with Zika.
Furthermore, Congenital Zika-associated syndrome is a set of anomalies (microcephaly) seen in infants born to mothers with a history of gestational Zika fever.
The U.S. CDC recommends that pregnant women and couples planning a pregnancy within the next three months consult with a healthcare provider before visiting an area reporting a Zika outbreak.
As of November 2023, there are no approved Zika vaccines.
In a Lancet Respiratory Medicine news article published on November 6, 2023, Sean O'Leary, MD, chair of the American Academy of Pediatrics (AAP)'s Committee on Infectious Diseases, stated the nationwide shortage of Beyfortus™ (Nirsevimab-alip), a newly approved respiratory syncytial virus (RSV) monoclonal antibody, could have been predicted.
"I would've predicted pretty high demand. I think probably too much was made of vaccine hesitancy and refusal..." wrote Dr. OLeary.
Sanofi, the producer of Beyfortus, stated on October 26, 2023, 'Despite an aggressive supply plan built to outperform past pediatric immunization launches, demand for this product, especially for the 100 mg doses used primarily for babies born before the RSV season, has been higher than anticipated.'
Sanofi collaborates closely with the U.S. Centers for Disease Control and Prevention (CDC) to ensure equitable distribution of available doses through the Vaccines for Children Program.
The CDC recently issued an advisory with recommendations for clinicians to prioritize 100-milligram doses for infants younger than six months and those with underlying medical conditions that predispose them to severe RSV.
Beyfortus is the second monoclonal antibody developed to prevent RSV in young children.
The AAP has recommended Arexis AB's palivizumab (Synagis) for high-risk infants and young children during an active RSV season.
Synagis was approved for initial use in the U.S. by the FDA in 1998. It is not an RSV vaccine but can help passively protect children with monthly dosing.
As of November 7, 2023, the RSV season began in Florida and has spread throughout the United States, impacting certain areas.

Clover Biopharmaceuticals, Ltd. announced today that it has completed the Biologic License Application (BLA) submission for its seasonal influenza vaccine (AdimFlu-S) to the Brazilian Health Regulatory Agency.
Upon approval, Clover will work with its local partner to commercialize AdimFlu-S, a quadrivalent split-inactivated vaccine containing hemagglutinin from four influenza virus strains (A and B).
If approved in Brazil, Clover's AdimFlu-S would have access to the Southern Hemisphere market.
Brazil is a vital vaccine market strategically. The country has the world's second-largest seasonal influenza vaccine market. Its market size is expected to surpass US$1 billion over the next five years.
"The BLA submission of AdimFlu-S in Brazil is another step towards our goal of becoming a global leader in the respiratory vaccine space and builds upon Clover's prior experience enrolling over 10,000 people in clinical trials across Brazil and South America," said Joshua Liang, Chief Executive Officer, and Executive Director of Clover, in a press release on November 6, 2023.
"By leveraging our unique globalization capabilities, we will continue expanding to other countries and regions to diversify our sales and maximize our impact on public health."
As of September 2023, AdimFlu-S has been listed in 26 provinces and municipalities in China.
Clover's diverse pipeline of candidates includes potential treatments that could significantly reduce the burden of vaccine-preventable diseases and make more diseases preventable.
In the United States, 145.42 million influenza vaccine doses (egg-based, nasal, cell-based) had been distributed in the U.S. as of October 28, 2023,

The World Health Organization's (WHO) 2023 Global Tuberculosis (TB) report, announced today, shows the impact of this centuries-old disease.
The report, published on November 7, 2023, features TB outbreak data from 192 countries and areas and shows that 7.5 million people were diagnosed in 2022, the highest figure recorded since 1995.
According to the WHO, an estimated 10.6 million people fell ill with TB in 2022, up from 10.3 million in 2021.
And the total number of TB-related deaths (including those among people with HIV) was 1.3 million in 2022. TB continues to be the leading killer among people with HIV.
Geographically, most people who developed TB in 2022 were in South-East Asia (46%), Africa (23%), and the Western Pacific (18%), with smaller proportions in the Eastern Mediterranean (8.1%), the Americas (3.1%) and Europe (2.2%).
In a press release, Dr. Tereza Kasaeva, Director of WHO's Global TB Programme, commented, "This report provides key data and evidence on the status of the TB epidemic and a review of the progress that serves to inform the translation of these targets and commitments into action in countries."
"We need all hands on deck to make the vision of ending TB a reality."
TB is a vaccine-preventable disease, with about 16 different Bacille Calmette-Guérin (BCG) vaccines in use globally.
In the U.S., access to the BCG vaccine is limited and considered for people who meet specific criteria. Merck's TICE® BCG vaccine is an attenuated, live culture preparation of the BCG strain of Mycobacterium Bovis and is available in 2023.

The World Health Organization (WHO) recently announced the Health Sciences Authority (HSA), Singapore; the Ministry of Food and Drug Safety (MFDS), Republic of Korea; and the Swiss Agency for Therapeutic Products (Swissmedic), Switzerland are the first three countries to be listed as WHO-Listed Authorities.
A WHO-listed authority (WLA) is a regulatory authority or a regional regulatory system that has been documented as complying with all the indicators and requirements specified by the WHO for the requested scope of listing based on an established benchmarking and performance evaluation process.
“This achievement is the result of investment by the Governments of the Republic of Korea, Singapore, and Switzerland in the strengthening of their regulatory systems and reaffirms the collaboration between WHO and the three Governments in promoting confidence, trust, and further reliance on authorities that have attained this global recognition, through the transparent and evidence-based pathway for designating and listing of WLAs,” said Dr. Yukiko Nakatani Assistant Director-General for Access to Medicines and Health Products, in a press release on October 31, 2023.
Implementing the WLA framework is intended to promote access to and supply of safe, effective, and quality medical products.
It is expected that HSA, MFDS, and Swissmedic will sustain this achievement, thereby enabling greater regulatory efficiencies and more informed decision-making at the national, regional, and global levels, wrote the WHO.