Search API
While most chikungunya virus (CHIKV) cases have been reported throughout the Americas in 2023, this mosquito-transmitted virus is causing outbreaks in about 115 countries.
Approximately 320,000 CHIKV cases and over 340 related deaths have been reported worldwide this year.
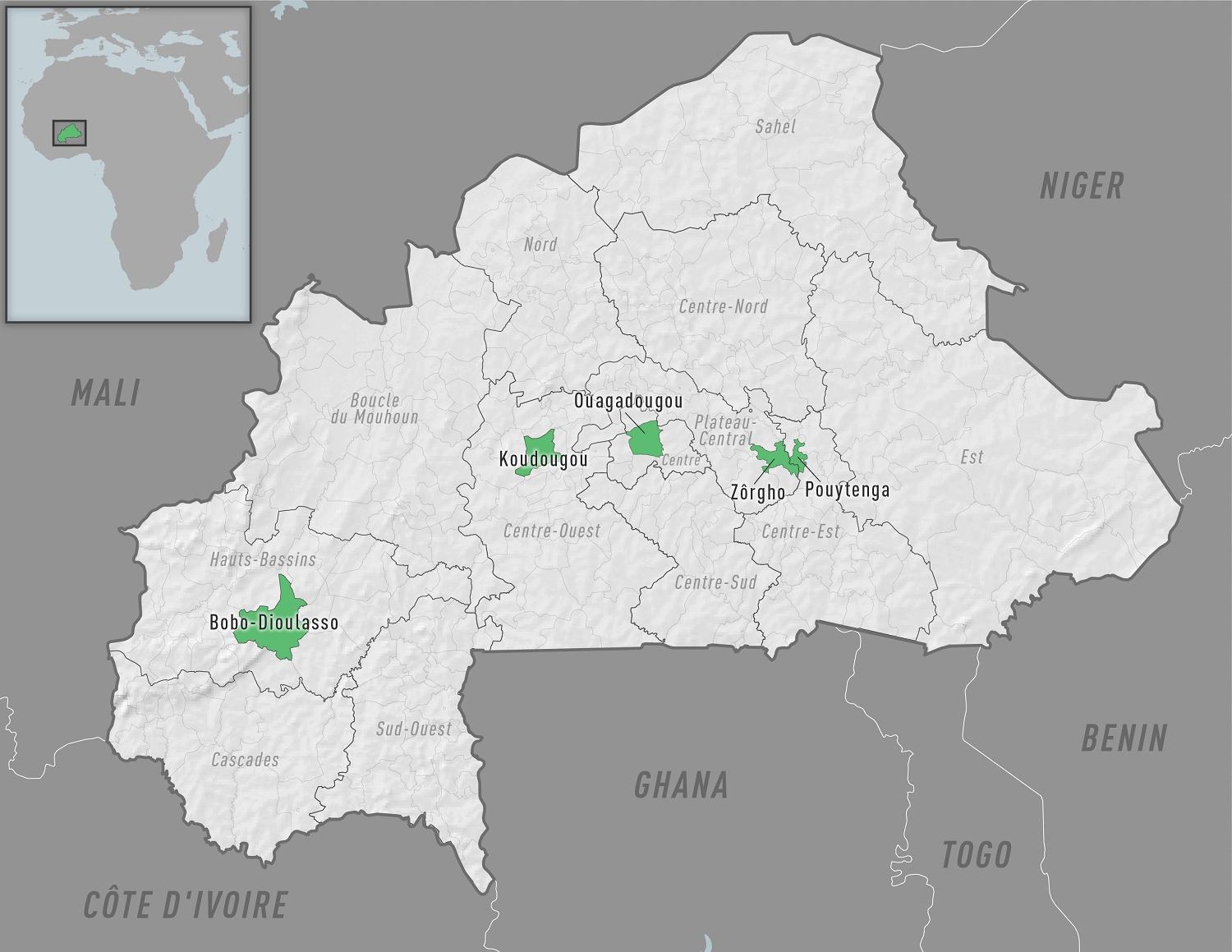
To alert international travelers, the U.S. Centers for Disease Control and Prevention (CDC) recently published a Level 2 - Practice Enhanced Precautions, Travel Health Advisory, regarding chikungunya outbreaks in Burkina Faso.
As of November 9, 2023, several districts have reported cases.
Burkina Faso Public Health Emergency Response Operations Center confirmed 89 chikungunya cases in Pouytenga in the Center-East region in September 2023.
Senegal, Burkina Faso's west African neighbor, has reported 210 chikungunya cases in 2023.
According to the CDC, chikungunya disease is caused by the virus and is spread to humans through mosquito bites.
If infected, you should seek medical care if you develop fever, joint pain, headache, muscle pain, joint swelling, or rash during or after travel.
If you are pregnant, reconsider travel to Burkina Faso, particularly if you are close to delivering your baby, says the CDC.
Mothers infected around the time of delivery can pass the virus to their baby before or during delivery. Newborns infected in this way are at risk for severe illness, including poor long-term outcomes.
Based on the U.S. Food and Drug Administration's recent approval of the IXCHIQ® Chikungunya Vaccine, Live (VLA1553), this disease can be prevented with a vaccine.
Other CHIKV vaccine candidates are conducting late-stage clinical trials in 2023.
Precedence Statistics today announced the global virology market size reached $2.6 billion in 2022 and is projected to reach $4.26 billion by 2032, expanding at a CAGR of 5.10%.
The U.S. virology market reached $690 million in 2022 and is projected to expand at a CAGR of 5.20%, reaching around $1.14 billion by 2032.
The virology market encompasses the study, diagnosis, treatment, and prevention of viral infections. It includes research, pharmaceuticals, diagnostic tests, and vaccines related to viruses like HIV, influenza, and hepatitis, which is a significant driver for the growth of the virology market.
The rapid mutation rates of many viruses pose hurdles for drug and vaccine development, necessitating ongoing research and adaptability.
The quest for effective vaccines to prevent viral diseases has also driven growth in this market. Government investments and public health initiatives have played a pivotal role in shaping the industry landscape.
The continuous evolution of vaccine technology, including mRNA and vector-based platforms, has broadened the scope of virology research.
Furthermore, the need for vaccine booster shots to combat emerging virus variants ensures a sustained demand for virology products.
Furthermore, the resumption of global travel contributes to the rapid spread of viruses, emphasizing the importance of virology in understanding, preventing, and managing infectious diseases worldwide.

The weekly National Immunization Survey (NIS) findings were published today, providing an update on the receipt of vaccination and intent for COVID-19, RSV, and influenza vaccination.
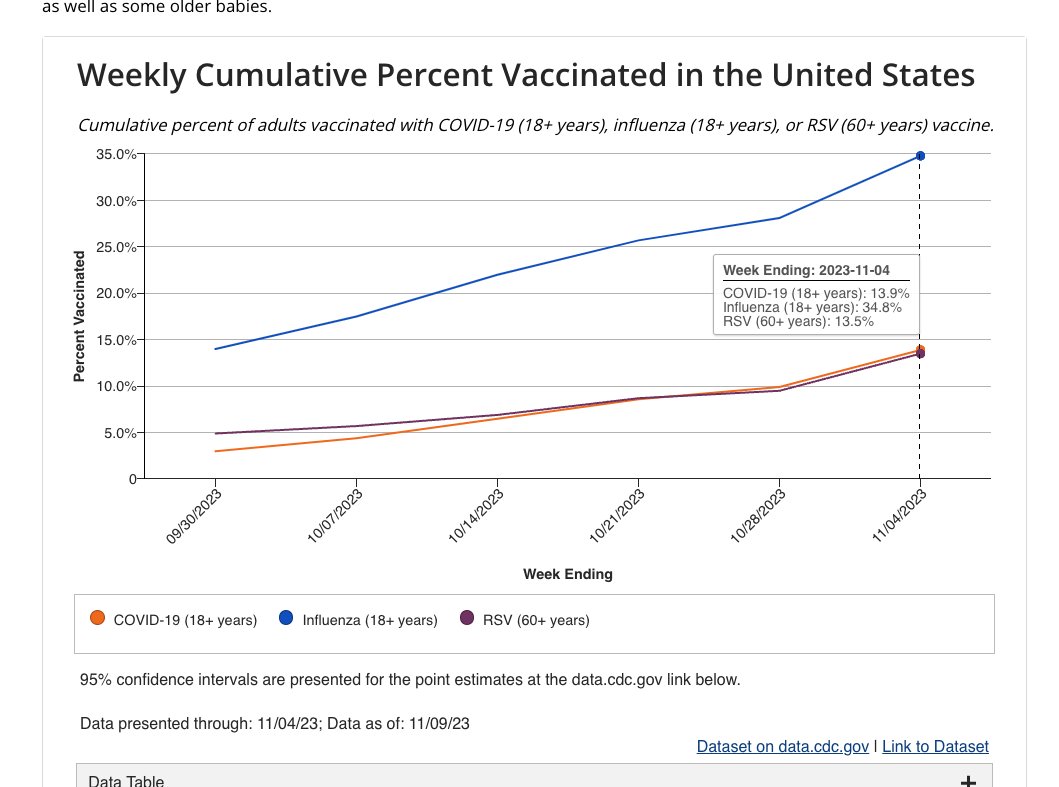
The NIS vaccination trend estimates increased as of November 13, 2023, are as follows:
The percent of the population reporting receipt of a flu vaccine is 32.6% (30.7-5%) for children and 34.8% (33.2-36.3%) for adults 18+, including 57.6% (53.7-61.5%) among adults 65+.
The percent of the population reporting receipt of the updated 2023-24 COVID-19 vaccine is 4.9% (95% confidence interval: 3.6-1%) for children and 13.9% (12.8-15.0%) for adults 18+, including 30.4% (26.9-33.9%) among adults 65+.
The percent of adults 60+ that report receiving an RSV vaccine is 13.5% (11.4-15.6%).
The U.S. CDC recommends that everyone six months and older stay current on COVID-19 and seasonal flu vaccines.
If you are 60 years and older, talk to your healthcare provider to see if RSV vaccination is proper for you.
The CDC also recommends Beyfortus™ (nirsevimab), a single-injection, monoclonal antibody product, providing passive immunization for all infants younger than eight months who are born during or entering their first RSV season and some older toddlers.
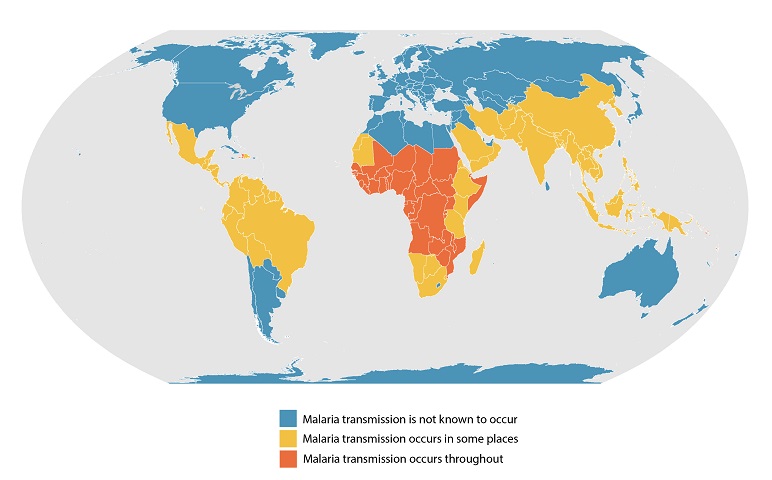
Despite decades of efforts to control malaria outbreaks, there were over 24 million cases in 2021. The African Region continues to shoulder the heaviest malaria burden, comprising 95% of patients globally, reports to the World Health Organization (WHO).
Unfortunately, children are particularly vulnerable during malaria outbreaks, as nearly half a million African children die from malaria every year.
In October 2023, the WHO recommended the programmatic use of malaria vaccines to prevent P. falciparum malaria in children living in endemic areas.
According to Gavi, the Vaccine Alliance, the approved malaria vaccines are delivering positive results.
On November 7, 2023, Gavi confirmed in a news article that the Mosquirix™ (RTS,S/AS01) malaria vaccine reduced mortality from all causes by 13% in children in the age group eligible for vaccination.
In addition to the significant reduction in all-cause mortality, the vaccine was also responsible for a 22% reduction in hospitalization for severe clinical malaria in children eligible for the vaccine.
"This means.....more children are going to be saved from death in Africa," commented John Bawa, PATH Malaria Vaccine Implementation Lead for West Africa.
As of November 2023, the Mosquirix vaccine is available in African countries, including Malawi, Kenya, and Ghana. Eighteen countries across different African regions are set to receive millions of doses over the next few years.
The Mosquirix vaccine was created in 1987 as part of a collaboration between GlaxoSmithKline and the Walter Reed Army Institute of Research that began in 1984.
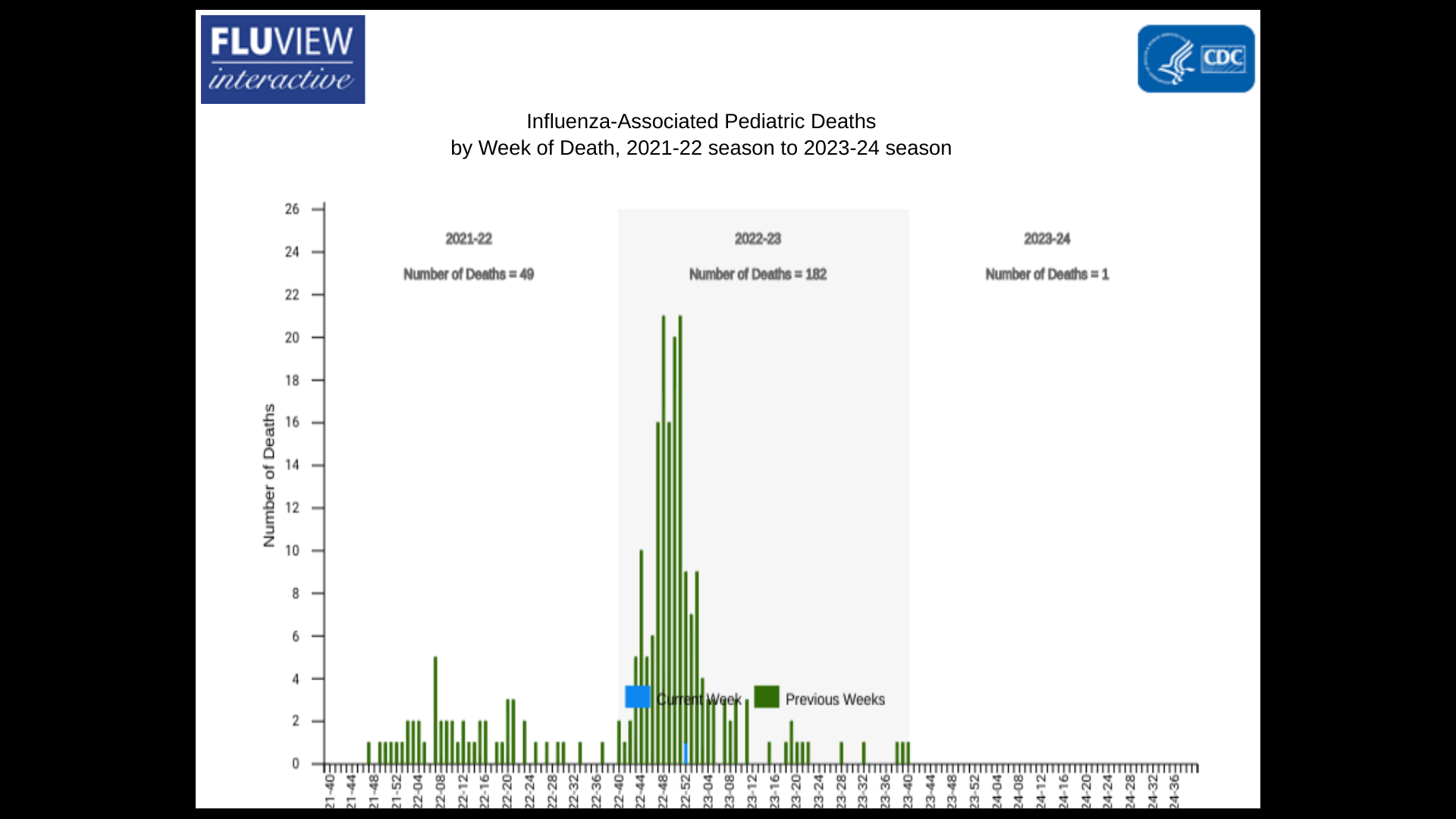
According to new data published today by the U.S. Centers for Disease Control and Prevention (CDC), influenza cases, hospitalizations, and deaths accelerated during the last week.
The CDC estimates that there have been at least 490,000 illnesses, 5,300 hospitalizations, and 330 deaths from influenza during the 2023-2024 flu season.
As of November 13, 2023, the CDC's Weekly U.S. Influenza Surveillance Report confirmed seasonal influenza activity increased in most parts of the country, most noticeably in the South Central, Southeast, and West Coast regions.
Additionally, the National Healthcare Safety Network Hospitalization Surveillance report for week #44 shows that 1,962 patients with laboratory-confirmed influenza were admitted to a hospital. The number of patients admitted to a hospital slightly increased compared to week #43.
Furthermore, weekly influenza-related deaths occurred during the following weeks: #44 (21), #43 (32), and #42 (43).
For the last flu season, the number of flu-related deaths reported by the National Center for Health Statistics totaled 9,697.
And the first influenza-associated pediatric death occurring during the 2023-24 season was recently reported.
A total of 182 influenza-associated pediatric deaths that occurred during the 2022-2023 season have been reported to the CDC.
With the Thanksgiving holiday fast approaching, the CDC's Director, Dr. Mandy Cohen, reminded everyone in a video posted on Facebook to make plans to get vaccinated.
The CDC also confirmed there are ample supplies of various flu shots this season.
As of November 4, 2023, over 147 million doses have been distributed.
Alzamend Neuro, Inc. today announced that it has submitted an investigational new drug ("IND") application to the U.S. Food and Drug Administration ("FDA") for the initiation of AL001-PTSD01, a Phase IIA plasma/brain pharmacokinetics clinical study of AL001, for treatment of patients with post-traumatic stress disorder (PTSD).
After receipt of a "study may proceed" communication from the FDA, Alzamend plans to initiate a Phase IIA study to characterize AL001 improvements of lithium levels in the brain compared to a marketed lithium salt in PTSD patients.
Alzamend anticipates that the new drug application ("NDA") development program for PTSD may, for safety, qualify for a 505(b)(2) NDA pathway to FDA approval, which can be available to new formulations of an approved drug.
"There are only two drugs approved by the FDA and currently available in generic form for PTSD patients," said Stephan Jackman, Chief Executive Officer of Alzamend, in a press release on November 13, 2023.
"Being able to develop a next-generation lithium product (AL001) that would not routinely require therapeutic drug monitoring (TDM) could positively impact the 9 million Americans afflicted with PTSD."
"We look forward to providing more details regarding the study's timeline and market opportunity in the near future."
Although lithium products do not have an FDA-approved indication for PTSD, case reports suggest that lithium treatment may be useful for treating PTSD patients. In particular, treatment with low doses (300–600 mg/day) of lithium carbonate has been reported to provide effective treatment in the reduction of inappropriate anger, irritability, anxiety, and insomnia in those patients.
The clinical observation of mood swings beyond the normal range, but milder than those associated with BD, reportedly suggested the presence of a sub-threshold mood disorder in these PTSD patients.
It has also been proposed that treatment of trauma with lithium to forestall the development of PTSD may be provided by pharmacological induction of mild transient amnesia.
Lithium was the first mood stabilizer approved by the FDA and is still a first-line treatment option for BD but is underutilized, perhaps because of the need for TDM, that is, routine monitoring of lithium drug levels in blood to help assure safety and effectiveness.
Lithium was the first drug that required TDM by regulatory authorities in product labeling because the effective and safe range of therapeutic drug blood concentrations is narrow and well-defined for treating BD when using lithium salts. Excursions above this range can be toxic, and below can impair effectiveness.
AL001 is a novel lithium-delivery system that has the potential to provide the benefits of marketed lithium salts while mitigating or avoiding currently experienced toxicities associated with lithium.
Results from Alzamend's recently completed Phase IIA multiple-ascending dose study of AL001 in Alzheimer's patients and healthy subjects identified a maximum tolerated dose ("MTD") that an independent safety review committee vetted.
This MTD is designed to distribute more lithium to the brain but at lower systemic exposure, resulting in an improved safety profile compared to currently marketed lithium salts.
This MTD was assessed to be unlikely to require TDM.
PTSD is a mental and behavioral disorder that can develop because of exposure to a traumatic event, such as sexual assault, warfare, traffic collisions, child abuse, domestic violence, or other threats to a person's life.
According to the NIH, about 3.6%, or roughly 9 million, adults in the U.S. have PTSD in a given year, and 9% of people develop it at some point in their life.
Children may also experience very stressful events that affect their thoughts and feelings.
In much of the world, rates for a given year are between 0.5% and 1% of the population.
As of November 13, 2023, the FDA has not approved an Alzheimer's disease-related vaccine candidate.
NOte: On Dec. 11, 2023, the headline was updated.

The Douglas County Health Department (DCHD) recently announced one confirmed active tuberculosis disease (TB) case at Westview YMCA, located near 156th and Ida Streets in the Bennington area in Omaha, Nebraska.
As of November 9, 2023, DCHD confirmed it is investigating more than 500 possible TB exposures that may have happened at the YMCA daycare. Those potential TB exposures could have occurred from late spring into late October 2023.
Children’s Nebraska has planned clinics to test children four years of age and under who were potentially exposed in the last ten weeks.
DCHD will hold clinics at Westview YMCA on November 15-17, 2023, to test anyone identified as exposed from late May until August 21, 2023.
County Health Director Dr. Lindsay Huse, MPH, DNP, RN, said in the news release that testing for TB is recommended only for those who had close contact on one or more occasions.
TB cases are relatively rare in Nebraska.
However, Douglas County confirmed 15 TB cases through September 2023 and had 15 confirmed cases in 2022.
Tuberculosis cases in the United States increased about 5% last year, led by California and Texas.
According to the U.S. CDC, TB is caused by a bacterium called Mycobacterium tuberculosis. The bacteria usually attack the lungs, but TB bacteria can attack any body part, such as the kidney, spine, and brain.
Not everyone infected with TB bacteria becomes sick. As a result, two TB-related conditions exist: latent TB infection (LTBI) and TB disease. If not treated properly, TB disease can be fatal, says the CDC.
While TB infections can be cured, it is also a vaccine-preventable disease.
Versions of the BCG vaccine have been used throughout the world for about 100 years.
According to a recent study, a seasonal analysis revealed that Brazil's most significant risk of Zika and Chikungunya disease occurs during the summer when higher temperatures occur.
The resurgence was identified in northeast Brazil between 2019 and 2021 for Zika and in 2021 for Chikungunya.
As of November 8, 2023, over 26,659 Zika cases have been reported in Brazil this year.
Published by the journal Scientific Reports on October 21, 2023, this trend is related to the increased Ae. aegypti mosquito infestation levels due to the decreased time for larval development.
And the increased proportion of infectious mosquitoes, given the decreased intrinsic incubation periods of the viruses in the vector.
Studies conducted in China, the United States, and the Brazilian states of Rio de Janeiro and São Paulo also indicated that temperature influenced the distribution patterns of Ae. aegypti and Ae. albopictus, consequently affecting the incidence of diseases they transmit.
The small but significant differences (from 0.7 to 2.6 ∘C) in the average temperature between the high-risk and no-risk areas for both diseases are worth consideration, wrote these researchers.
For example, Banu et al. showed that an increase of 1 ∘C could be related to a future rise in arbovirus disease cases.
In recent decades, consistent and widespread warming has been observed throughout Brazil, with greater extreme heat occurring during spring and summer.
From a prevention perspective, Zika vaccine candidates continue in clinical trials. However, the U.S. FDA recently approved the first Chikunynga vaccine.