Search API
The Denpasar City Agriculture Service recently announced villages/sub-districts in Bali Province to form a Rabies Alert Team to suppress cases of rabies transmission in the capital city of Bali Province.
It is estimated that there are 82,195 rabies-transmitting animals in Denpasar City, Indonesia, as of September 2023.
Announced on November 21, 2023, the Head of the Denpasar City Agriculture Service, A.A. Bayu Brahmasta, informed reporters, “The Rabies Alert Team will be tasked with providing education and outreach to the community.”
This news comes from TheBaliSun, which reported two additional residents who live near Keramas Beach were bitten by a dog that has gone on to test positive for rabies.
According to the U.S. CDC, dog rabies outbreaks have been active in Bali since 2008.
Bali is an often-visited destination, hosting over 4.8 million international visitors over the past year.
Each year throughout the world, rabies, a viral disease of mammals, kills approximately 50,000 people, primarily children. It is almost always spread by an animal bite but can also be spread when a rabid animal’s saliva gets directly into the eyes, nose, mouth, or broken skin.
The primary source of human infection worldwide is dogs. However, in the U.S., bats are the source of most rabies infections in 2023.
The CDC says that if your activities bring you into contact with animals, you should consider pre-exposure rabies vaccination, a multi-dose series given before departure overseas.
Even if you receive pre-exposure vaccination, you will still need immediate medical treatment if you are bitten or scratched by an animal.
The good news is human rabies is rare in the U.S., averaging about three cases annually since 2000.
In the U.S., rabies vaccines are available in 2023.

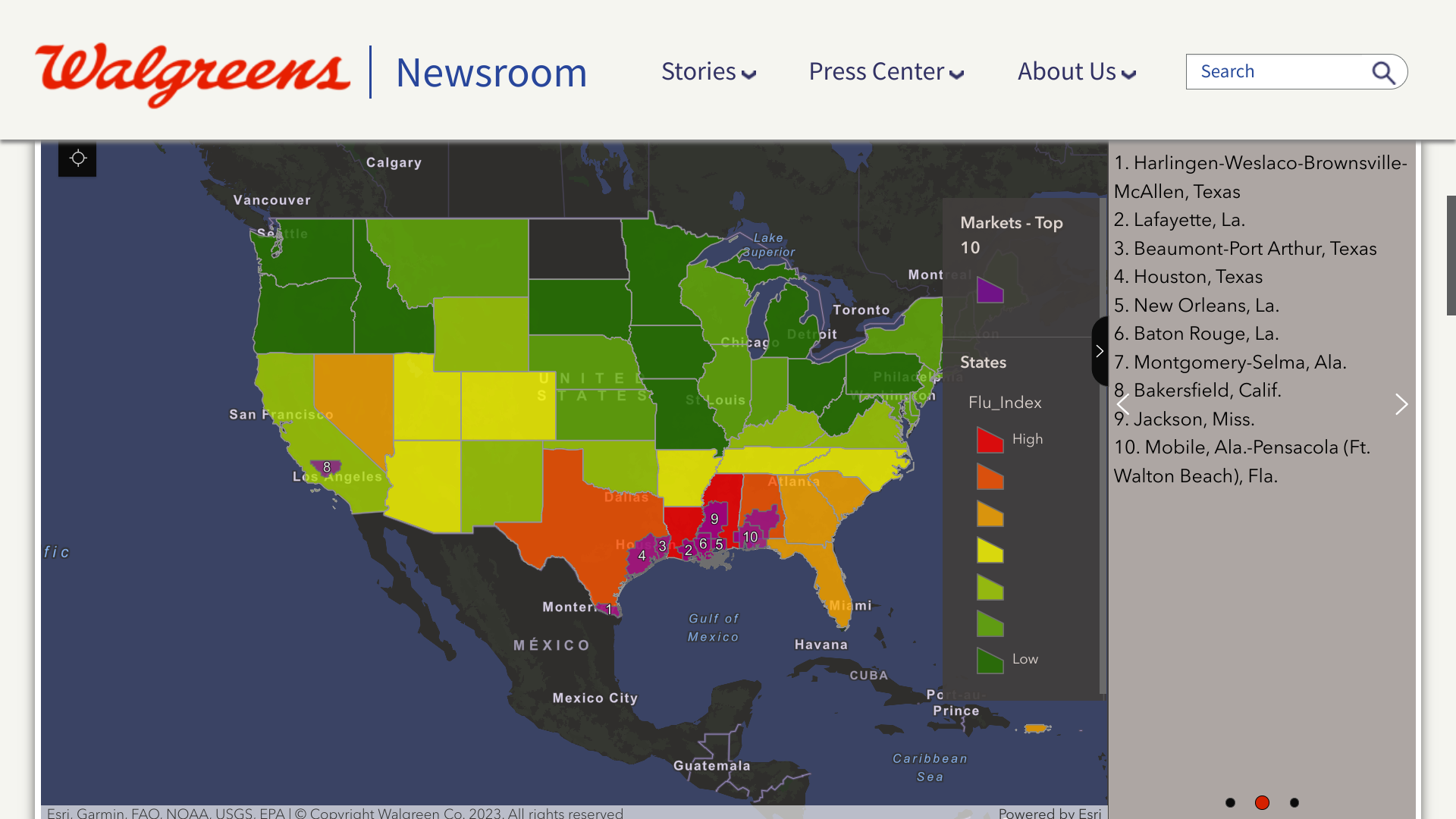
The Walgreens Flu Index® recently coded the top 10 United States cities experiencing the most influenza detections. The Index shows flu activity is 50% higher than last week.
As of November 18, 2023, the updated listing includes cities primarily located in the south-central U.S.:
- Harlingen-Weslaco-Brownsville-McAllen, Texas
- Lafayette, La.
- Beaumont-Port Arthur, Texas
- Houston, Texas
- New Orleans, La.
- Baton Rouge, La.
- Montgomery-Selma, Ala.
- Bakersfield, Calif.
- Jackson, Miss.
- Mobile, Ala.-Pensacola (Ft. Walton Beach), Fla.
While various flu shots are readily available at most clinics and pharmacies in October 2023, about 15% of individuals who received their flu shot at Walgreens were millennials, and only 9% were Gen Z.
The Walgreens Flu Index® is an online, interactive tool showing the most significant increases in flu activity week-over-week.
Shanghai Ark Biopharmaceutical Co., Ltd. (ArkBio) today announced that it had received IND approval from the National Medical Products Administration in China for a novel monoclonal antibody AK0610 that was bioengineered based on a respiratory syncytial virus (RSV) infection.
AK0610 specifically targets the RSV pre-F protein.
It demonstrated potent neutralizing effects against RSV both in vitro and in vivo. With a prolonged half-life, it holds promise as a next-generation long-acting antibody for RSV prevention.
Dr. Jim Wu, chairman and CEO of ArkBio, commented in a press release on November 23, 2023, "We are excited with the IND approval of AK0610 and its great potential in the field of RSV prevention."
"...We will strive to provide very needed RSV high-risk population and patients with efficacious prevention and treatment solutions."
The discovery and pre-clinical characterization have been published in hLife jointly by Professor George Fu Gao and his team from the Institute of Microbiology, Professor Zhengde Xie and his team from Beijing Children's Hospital, Capital Medical University, National Center for Children's Health, China, and ArkBio R&D team.
China has one of the highest rates of lower respiratory tract infection in children caused by RSV, accounting for 18-27% of all hospitalizations in children under five years old due to RSV infections.
ArkBio licensed AK0610 intellectual properties from the Institute of Microbiology, Chinese Academy of Sciences and Beijing Children's Hospital, Capital Medical University, National Center for Children's Health, China, followed by further engineering and optimization at ArkBio.
Currently, there is no approved drug for preventing RSV infection in China.
In the United States, RSV antibody passive immunization products have been U.S. FDA-approved since 1998.
Recently, AstraZeneca and Sanofi co-developed Beyfortus™ monoclonal antibody, which was approved by the FDA to protect infants through their first and second RSV season.

SK bioscience today announced it is partnering with Hilleman Laboratories Singapore to develop a low-cost, improved manufacturing process, second-generation Ebola-Zaire vaccine.
Currently, Ebola vaccines have been authorized and used in Africa since 2019.
On November 22, 2023, SK bioscience confirmed it will acquire unique expertise and know-how for the use of recombinant Vesicular Stomatitis Virus Vector (rVSV) technology platform in close collaboration with Hilleman Laboratories to potentially jointly develop other vaccines against a variety of viral infectious diseases.
Jaeyong Ahn, CEO of SK bioscience, commented in a press release, "Developing a vaccine to prevent viruses causing diseases with a high fatality rate, such as Ebola-Zaire, is essential for us to protect humanity."
"By cooperating with Hilleman Laboratories for a successful development of the second-generation Zaire Ebolavirus vaccine, we will contribute to overcoming the Ebola Zaire disease burden and expand our cooperation with global companies and institutions."
In 2014, the World Health Organization declared an international public health emergency during the Ebola outbreak and encouraged the development of the vaccine when the virus was spreading rapidly in West Africa.
Ebola Virus Disease is a rapidly progressive, severe, and transmissible hemorrhagic illness caused by infection with one of the Ebola Virus (EBOV) species. While there are six identified EBOV species, the Zaire Ebola virus strain has been the leading cause of outbreaks over the last 20 years.
Ever since the Ebola virus was first discovered in 1976, there have been multiple outbreaks resulting in significant loss of lives (50% mortality rate) and economic impact.
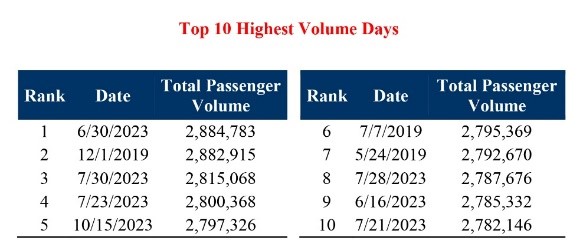
The U.S. Transportation Security Administration (TSA) has screened a record number of passengers in 2023 and anticipates airport security checkpoints nationwide will be busier than ever this holiday travel season.
The season kicked off with Thanksgiving travel, which concludes November 28, 203.
Historically, the Sunday following Thanksgiving will likely be the busiest travel day. The TSA expects to screen 30 million passengers this season.
TSA Administrator David Pekoske recently stated, "We are ready for the anticipated volumes and are working closely with our airline and airport partners to ensure we are prepared for this busy holiday travel season."
"We will also do our best to maintain wait time standards of under 10 minutes for TSA PreCheck® lanes and under 30 minutes for standard screening lanes."
"I am grateful for our dedicated employees who remain vigilant and focused on the mission during this holiday travel season and beyond."
The U.S. Department of State and the U.S. CDC publish notices to alert international travelers to various risks.
Furthermore, certain travel vaccinations are recommended before visiting disease outbreak areas, such as Dengue and Zika.
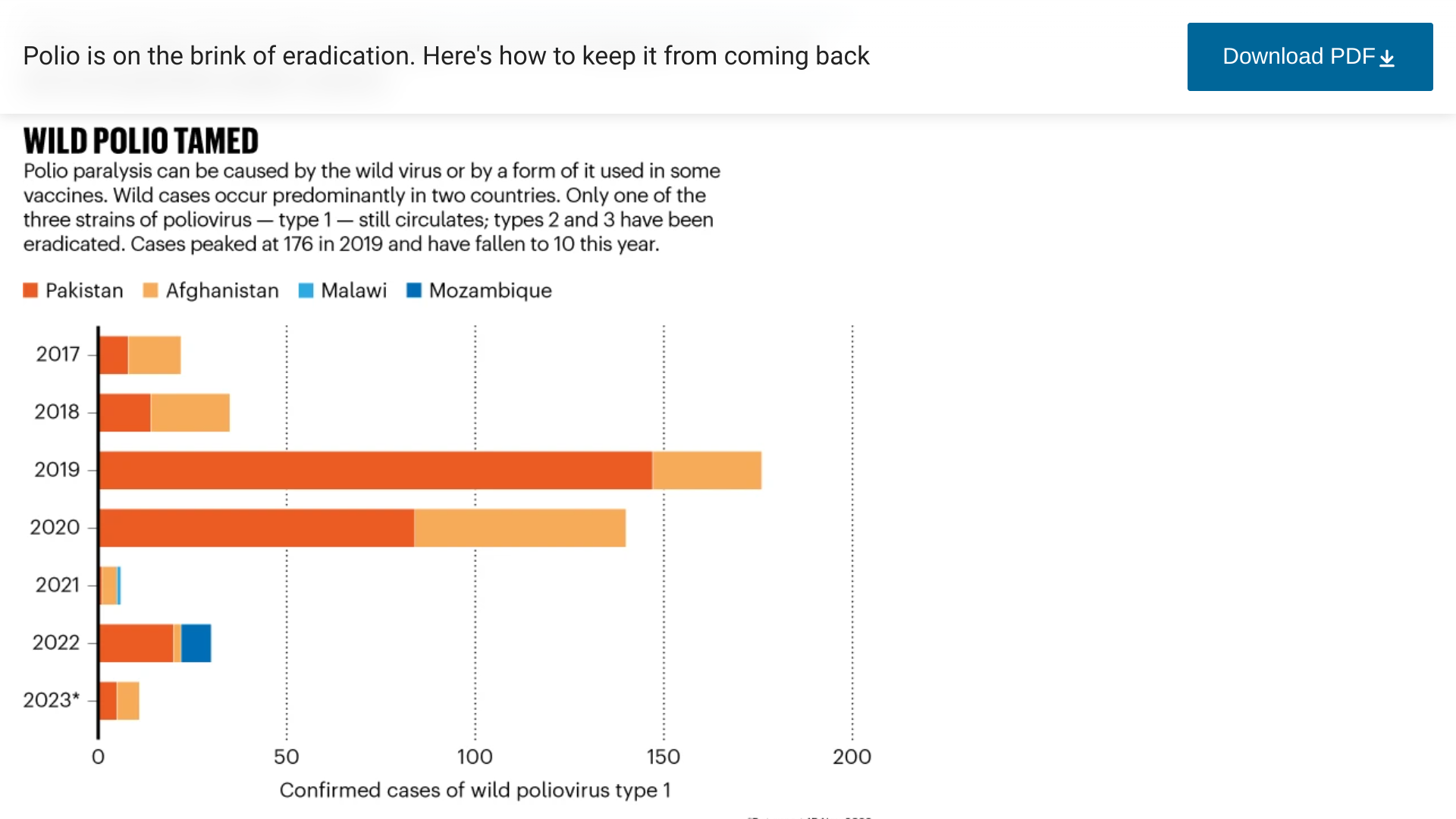
The campaign to eradicate polio could succeed in the next few years. But that's just the beginning of a new challenge, keeping it away forever, wrote Aisling Irwin in an article published by the journal Nature on November 21, 2023.
With the demise of the poliovirus in sight, health authorities are planning what happens next.
In 1988, the World Health Organization (WHO) passed a resolution to eradicate polio.
However, a WHO committee announced in August 2023 that although encouraged by the reported progress, the risk of the international spread of poliovirus remains a Public Health Emergency of International Concern, and the extension of Temporary Recommendations for a further three months was recommended.
The WHO committee urged the polio program to strengthen all aspects of surveillance, noting that significant gaps remain in many affected countries.
That conclusion may be because achieving polio eradication is not extinction.
Polio could lurk in testing labs and manufacturing facilities — from which it has previously leaked — and even in some people. Mistakes years after eradication could let polio into an unprotected population where it could "wreak havoc.
The end of polio is only the beginning of another effort: developing the resilience to keep it away, says Liam Donaldson, a public health specialist at the London School of Hygiene & Tropical Medicine, UK, and the lead author of a series of independent reports on the campaign's progress.
"People have signed up for polio eradication, but they've not signed up for the longer journey."
The WHO's 6th Report of the Polio Transition Independent Monitoring Board is posted at this link, and current polio outbreaks are listed by Precision Vax.
The World Health Organization (WHO) today announced that 331,200 doses of Mosquirix™ RTS, S/AS01, arrived in the Republic of Cameroon.
Mosquirix is a recombinant malaria vaccine with the P. falciparum circumsporozoite protein.
The delivery on November 21, 2023, is the first to a country located on the Gulf of Guinea not previously involved in the WHO malaria vaccine pilot program and signals that scale-up of vaccination against malaria across the highest-risk areas on the African continent will begin shortly.
A further 1.7 million doses of Mosquirix are expected to arrive in Burkina Faso, Liberia, Niger, and Sierra Leone in the coming weeks, with additional African countries set to receive doses in the months ahead.
Since 2019, Ghana, Kenya, and Malawi have been administering the vaccine in a schedule of 4 Mosquirix doses from around five months of age in selected districts as part of the pilot program known as the Malaria Vaccine Implementation Programme (MVIP).
More than 2 million children have been reached with the malaria vaccine in three African countries through MVIP – resulting in a 13% drop in all-cause mortality in children age eligible to receive the vaccine and substantial reductions in severe malaria illness and hospitalizations.
Nearly every minute, a child under five dies of malaria in Africa.
In 2021, there were 247 million malaria cases globally, which led to 619,000 deaths in about 84 countries.
Of these deaths, 77% were children under five years of age.
Approximately 95% of global malaria cases and 96% of related deaths in 2021.
The U.S. Centers for Disease Control and Prevention (CDC) has issued various alerts for malaria-endemic countries, including Costa Rica.
The CDC has recently confirmed autochthonous (local) malaria cases in Florida (seven), Texas, Maryland, and Arkansas.
As of November 22, 2023, malaria vaccines are unavailable in the U.S.
According to a statement issued today by the Minnesota Department of Health, healthcare providers should not inadvertently give infants adult respiratory syncytial virus (RSV) vaccines.
As of November 20, 2023, the U.S. CDC has received several reports of this happening when the infant should have received Beyfortus™ (Nirsevimab-alip), the first U.S. FDA approved extended half-life monoclonal antibody (mAb) offering passive immunization to prevent lower respiratory tract infections caused by the RSV.
Vaccine providers who carry both Beyfortus and RSV vaccines (Abrysvo or Arexvy) should be especially diligent in following vaccine administration safety procedures to prevent errors.
To minimize the risk of errors, store RSV vaccines and mAb in their original packaging on different shelves and clearly label them.
If an RSV vaccine is administered to an infant, immediately inform the provider and the parent/guardian of the error.
Furthermore, promptly report the error to the Vaccine Adverse Event Reporting System - (www.cdc.gov/vaccinesafety/ensuringsafety/monitoring/vaers/index.html).
And send an email to [email protected] for guidance on the next steps.

JUVE Patent today reported the European Patent Office Opposition Division has revoked an essential mRNA vaccine patent.
As of November 21, 2023, a contested mRNA patent owned by Moderna Inc. was declared invalid.
Moderna believes Pfizer and BioNTech copied features of the company's patented technologies critical to the success of mRNA vaccines.
According to Reuters, BioNTech welcomed the decision, calling the patent office's decision "an important one, as we believe that this and others of Moderna's patents do not meet the requirements for grant and should never have been granted."
The legal dispute revolves around Moderna's patents EP 3 590 949 B1 and EP 3 718 565 B1, which protect "ribonucleic acids containing n1-methyl-pseudouracils and uses thereof" and "respiratory virus vaccines," respectively.
However, while both patents are concerned with mRNA vaccines, they have different applications.
EP 949 is concerned with claims concerning modified mRNA.
And EP 565 covers the "betacoronavirus mRNA-LNP vaccine," an improved substance for the prevention of contracting COVID and other respiratory diseases.
The unedited news article is posted at this link.

