Search API
As the United States heads into the winter months that typically coincide with peak respiratory illness season, Walgreens provides in-store and at-home testing and treatment options to help everyone stay healthy and feel better faster if they are experiencing symptoms.
"Respiratory illness activity and hospitalizations are picking up in many parts of the U.S., and these numbers are likely to continue increasing in the coming months," said Anita Patel, PharmD, Vice President of Pharmacy Service Development at Walgreens, in a press release on December 7, 2023.
"In addition to staying up to date on your vaccinations, getting tested, seeking treatment promptly, and practicing good respiratory etiquette are all important steps to protect yourself and your loved ones this winter, especially if you are feeling sick or planning to travel and gather for the holidays."
Walgreens stated testing is the best way to know if you have a specific respiratory virus so you can take appropriate precautions and get the proper relief or treatment immediately.
With influenza, RSV, and COVID-19 viruses circulating, visiting a local pharmacist in 2023 may be just what the doctor ordered.
Flu and RSV vaccines are covered by most insurance plans with a $0 copay and by Medicare and Medicaid in certain states.
Dr. Patel added, "People are increasingly relying on pharmacies as a one-stop destination for these services and deepening their relationships with their community pharmacists, who work tirelessly to provide the care and information they need all season long."
"As the nature of respiratory illness season continues to evolve post-pandemic, we remain focused on being a trusted partner in keeping our communities healthy."
As of December 2, 2023, the Walgreens Flu Index listed the top three states indicating influenza outbreaks:
- Louisiana
- Mississippi
- Texas
With nearly 9,000 retail locations across America, Puerto Rico, and the U.S. Virgin Islands, Walgreens is proud to be a neighborhood health destination serving almost 10 million customers daily.
Various types of flu shots are available as of December 7, 2023.

The approval of yet another RNA-based vaccine might not seem momentous, wrote Elie Dolgin in an article published by the journal Nature.
But the endorsement last week by Japanese authorities of a jab against the SARS-CoV-2 coronavirus constructed using a form of RNA that can make copies of itself inside cells — the first 'self-amplifying' RNA granted full regulatory approval anywhere in the world — marks a pivotal advance.
The December 6, 2023, article is posted at this link.
In late November 2023, CSL and Arcturus Therapeutics announced that Japan's Ministry of Health, Labor, and Welfare approved ARCT-154, a self-amplifying Messenger RNA COVID-19 vaccine for initial vaccination and booster for adults 18 years and older.

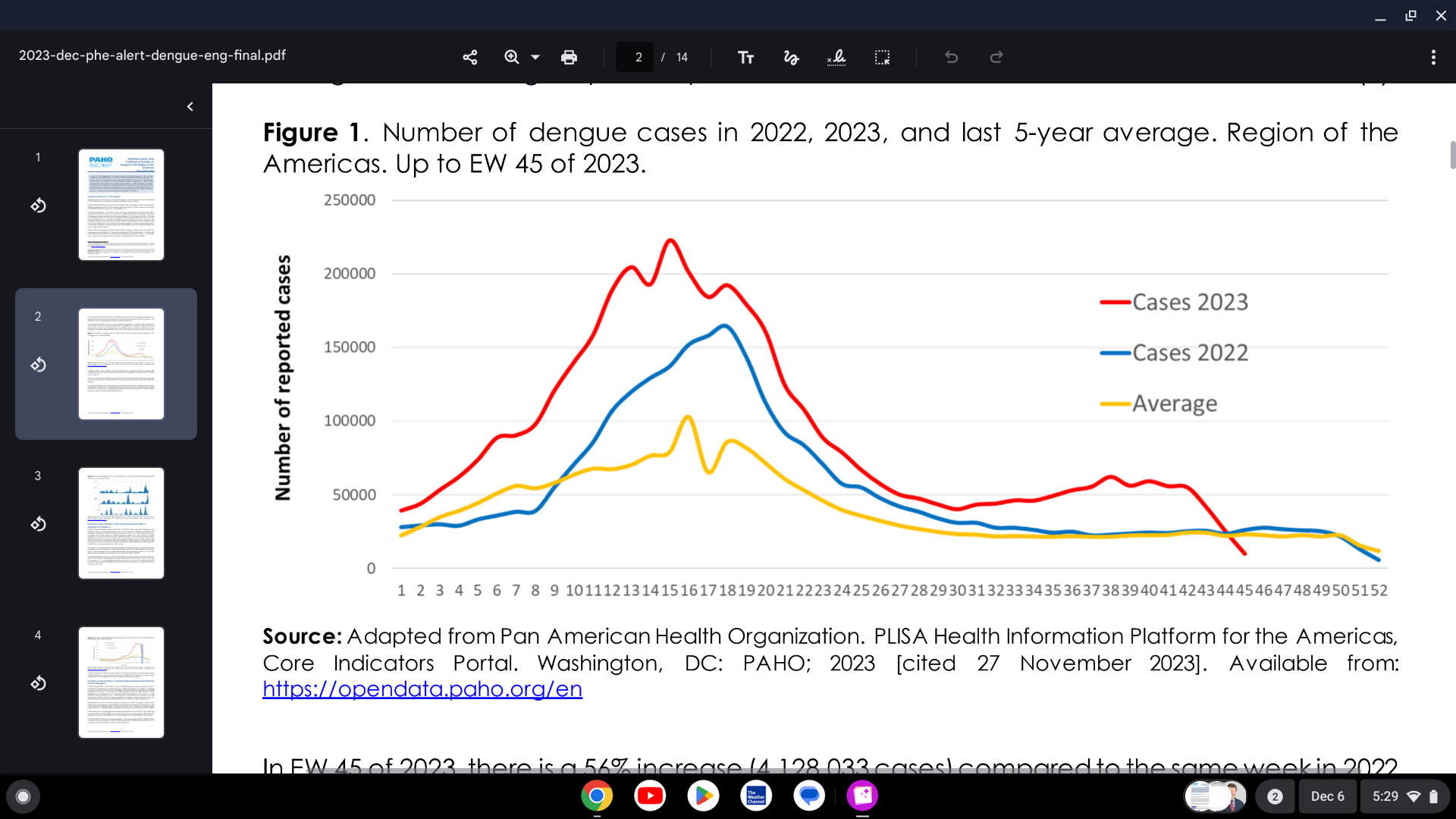
The Pan American Health Organization (PAHO) issued an Epidemiologic Alert confirming a significant increase in dengue during the second half of 2023 in several countries of the Region of the Americas, especially in Central America and the Caribbean.
As of December 5, 2023, the Americas set a new record number of dengue cases with over 4.1 million patients.
According to the seasonal pattern of dengue and the current rainy season, the PAHO called for the intensification of preparedness actions within healthcare services to facilitate access and proper management of patients ahead of the Southern Hemisphere's peak season.
In 2023, through week #45, 1,954 dengue-related deaths were reported in the Region of the Americas (case fatality rate [CFR]: 0.048%).
The most significant number of dengue cases were reported in Brazil, with 2,909,404 cases.
Of the 6,340 severe dengue cases reported in 2023, the highest numbers were recorded in Brazil with 1,474 cases, Colombia with 1,390, Mexico with 1,142, Peru with 1,065, and Bolivia with 640 cases.
In addition, a notable increase in the notification of locally transmitted cases has been observed in places such as The Bahamas and the state of Florida.
Given this situation, the PAHO / World Health Organization urges Member States to implement appropriate actions at the level of patient care services, including triage, diagnosis, and timely and proper treatment of dengue cases and other arboviruses such as Zika.
As of December 2, 2023, the PAHO indicated 31,780 Zika cases across the Americas this year.
From a protection perspective, two dengue vaccines have been authorized in various countries since October 2022.
The U.S. CDC's Weekly COVID-19 Vaccination Dashboard estimates vaccinations and intent for vaccination using various data sources, including surveys, healthcare claims, electronic medical records, and immunization information systems data.
As of December 6, 2023, category highlights include the following data:
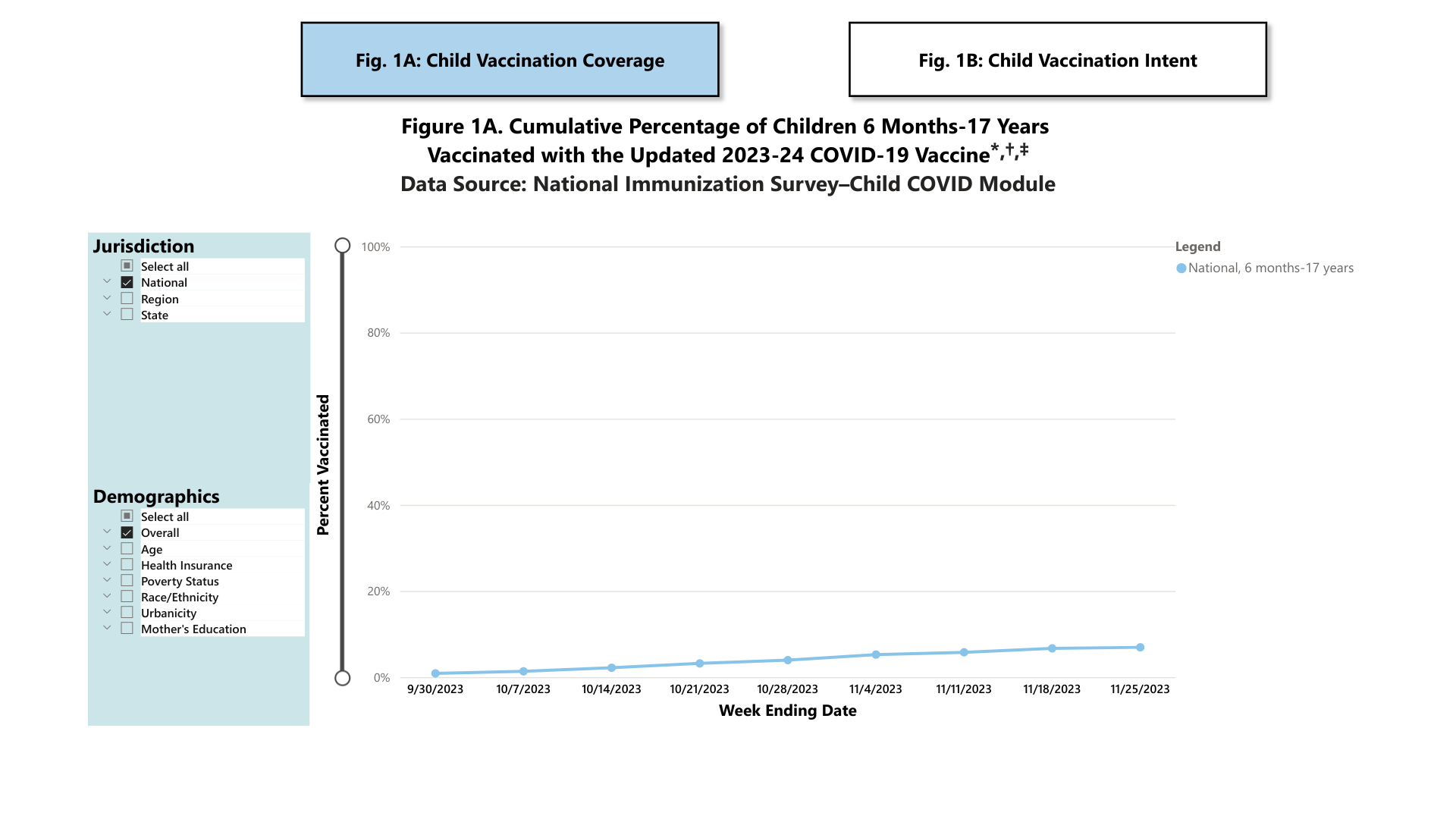
As of November 25, 2023, 6.9% (95% Confidence Interval: 6.0%-7.9%) of children were reported to be up-to-date with the 2023-24 COVID-19 vaccine.
About 17.3% of children had a parent who reported they planned to get their child vaccinated. Additional COVID-19 vaccination data for children by demographic characteristics at the national level and overall estimates by jurisdiction are available at this CDC link.
For pregnant women, 8.9% had received the updated 2023-24 COVID-19 vaccine.
Vaccination coverage was highest among non-Hispanic Asian (15.2%) women and lowest among non-Hispanic Black (2.7%) pregnant women.
And for adults, about 16% reported having received an updated 2023-24 COVID-19 vaccine since September 14, 2023. Vaccination coverage increased by age and was highest among adults 65 and older [33.3%, (31.2%-35.3%)].
From a geography perspective, the District of Columbia reported the most significant number of vaccinated adults, with about 30.7%.
COVID-19 vaccination coverage estimates among all adults are based on CDC's National Immunization Survey–Adult COVID Module data.
Recent Highly Pathogenic Avian Influenza (HAPI) outbreaks in Europe have led France to issue a 'high' alert, forcing poultry farms to keep birds indoors as of December 5, 2023.
The French government wrote, 'continuing strong dynamic of HPAI virus infection recorded in Europe, while the first contamination of a farm had been detected a few days ago in France, has now led the public authorities to place all of the territories at high-risk levels with of HPAI.'
The French Ministry of Agriculture and Food Sovereignty launched a vaccination campaign in October 2023 to reduce the spread of HAPI. The mandatory vaccination of domestic ducks applies to all of Metropolitan France (except Corsica Island).
To better inform the public, the Ministry has published 'Ten Things to Remember About HAPI Vaccination' (posted in French) on November 17, 2023.
Avian influenza, known as bird flu, is harmless in cooked food and spreads among various types of birds, mammals, and even to humans.
Furthermore, the World Health Organization report #907 confirmed sporadic influenza A(H5N1) clade 2.3.4.4b virus detections in humans.
On September 29, 2023, the U.S. government announced that it restricted the import of poultry from France and its European Union trading partners following France's decision to vaccinate meat ducks against HPAI.
Additionally, the U.S. Centers for Disease Control and Prevention (CDC) published a Technical Report in 2023 that confirmed the overall risk to human health associated with the ongoing outbreaks of highly pathogenic A(H5N1) viruses remains low.
From an outbreak protection perspective, the CDC confirmed in June 2023 that about 20 million H5N1 and 12 million H7N9 vaccines for humans were available in the U.S. National Strategic Stockpile.
Note: This article was updated on Dec. 7, 2023.

Novavax, Inc. today announced that Health Canada has granted expanded authorization for Nuvaxovid™ XBB.1.5 Vaccine (Recombinant protein, Adjuvanted) for active immunization to prevent COVID-19 caused by the SARS-CoV-2 coronavirus in individuals aged 12 and older.
The Public Health Agency of Canada's National Advisory Committee on Immunization recommended XBB COVID-19 vaccines that target more recent, immune-evasive virus variants.
The expanded authorization was based on non-clinical data showing that Novavax's COVID-19 vaccine induced functional immune responses against XBB.1.5, XBB.1.16, and XBB.2.3 variants.
Additional non-clinical data demonstrated that Novavax's vaccine-induced neutralizing antibody responses to subvariants BA.2.86, EG.5.1, FL.1.5.1, and XBB.1.16.6 as well as CD4+ polyfunctional cellular (T-cell) responses against EG.5.1 and XBB.1.16.6.
These data indicate that Novavax's vaccine can stimulate both arms of the immune system and may induce a broad response against currently circulating variants.
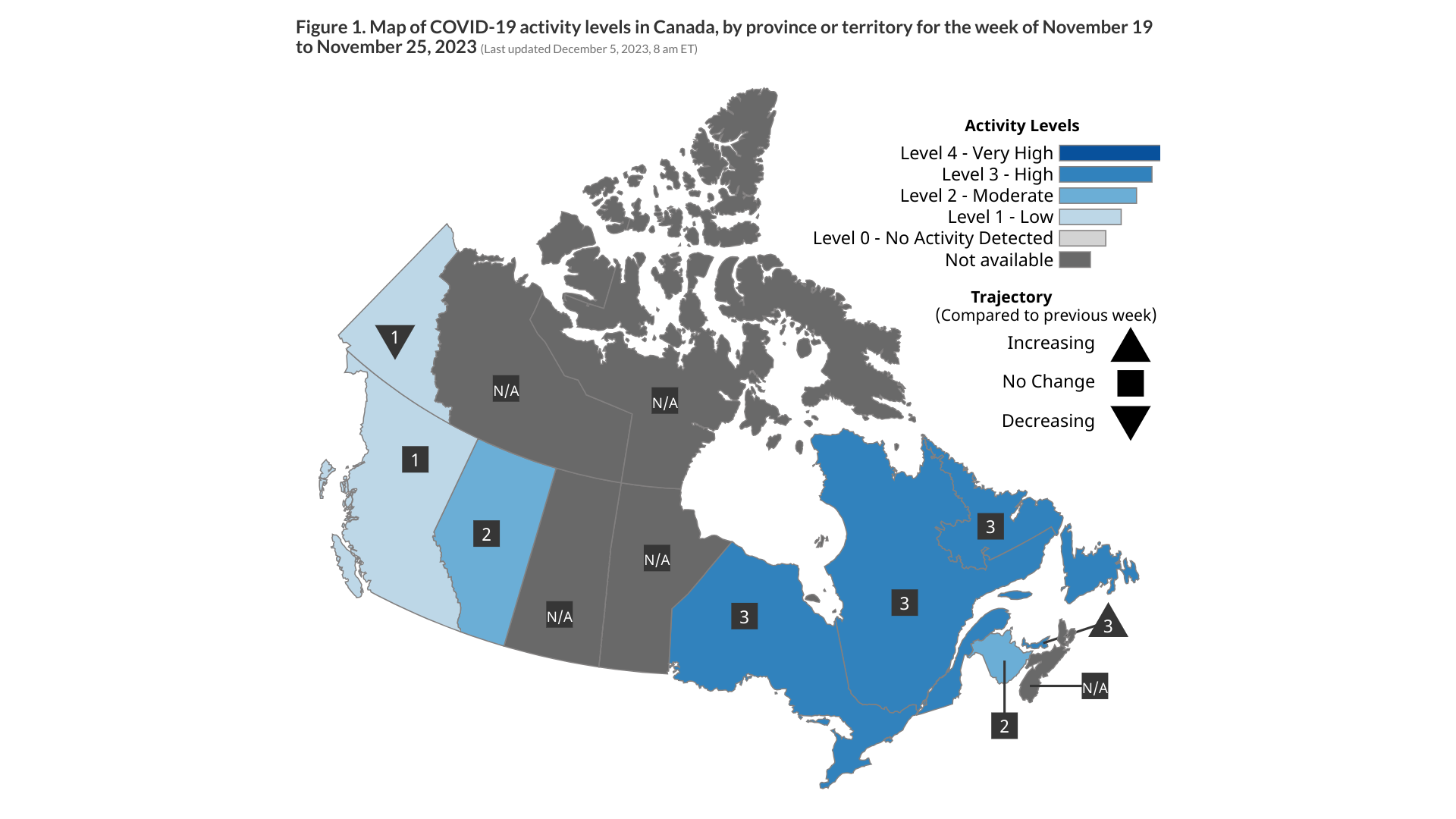
In Canada, recombinant XBB sub-lineages remain dominant, representing 93% of sequences in the past month. HV.1, HK.3, and BA.2.86 (including JN.1) are the major lineage groups demonstrating consistent growth in Canada.
Health Canada's latest COVID-19 numbers were updated at this link as of December 5, 2023, 8 am ET.
"Today's expanded authorization will support the Canadian government's strong commitment to provide its citizens with effective options, such as our protein-based non-mRNA vaccine, in the campaign against currently circulating COVID-19 variants," said John C. Jacobs, President and Chief Executive Officer, Novavax, in a press release on December 5, 2023.
Novavax's updated COVID-19 vaccine is also authorized in the U.S. and Europe by the World Health Organization and is under review in other markets.
Vaxcyte, Inc. today announced the publication of the results from the VAX-24 vaccine candidate's pneumococcal disease (PD) proof-of-concept study in the journal The Lancet Infectious Diseases.
This phase 1/2 clinical trial evaluated the safety, tolerability, and immunogenicity of Vaxcyte's investigational 24-valent, carrier-sparing pneumococcal conjugate vaccine (PCV) compared to the current standard-of-care, Prevnar 20® (PCV20, APEXXNAR), for the prevention of invasive pneumococcal disease (IPD) in healthy adults.
The study results showed that VAX-24 demonstrated a safety and tolerability profile comparable to PCV20 at all doses studied and an immunogenicity profile that met or exceeded established regulatory immunogenicity standards for all 24 serotypes at the conventional 2.2 mcg dose.
"The results from the proof-of-concept study provided the first look at the safety and immunogenicity profile of VAX-24 in adults, giving us confidence in the 2.2 mcg dose we plan to advance into Phase 3," said Dr. Jakub Simon, Chief Medical Officer of Vaxcyte, in a press release on December 4, 2023.
"We look forward to initiating our Phase 3 pivotal, non-inferiority study, designed to further establish the clinical potential of VAX-24, and announcing topline data, which we expect in 2025."
PD is an infection caused by Streptococcus pneumoniae bacteria, says the U.S. CDC.
It can result in IPD, including meningitis and bacteremia, and non-invasive PD, including pneumonia, otitis media, and sinusitis.
People can get pneumococcal disease more than once. A previous pneumococcal infection will not protect you from future infection. Therefore, CDC recommends pneumococcal vaccination even if someone has had pneumococcal disease in the past.
In the United States, approximately 320,000 people get pneumococcal pneumonia each year, which is estimated to result in about 150,000 hospitalizations and 5,000 deaths.
Pneumococci also cause over 50% of all cases of bacterial meningitis in the U.S.
As of December 2023, several approved pneumococcal vaccines are available at clinics and pharmacies in the U.S.

Osivax today announced that it has received a grant of over $1.5 million from the U.S. National Institute of Allergy and Infectious Diseases (NIAID).
The grant will support preclinical studies evaluating the breadth of protection and immune response induced by OVX836 against pandemic influenza strains.
OVX836 is a first-in-class influenza vaccine candidate that targets the nucleoprotein (NP), a highly conserved internal antigen. Unlike surface antigens, the NP is much less likely to mutate, providing a broader and more universal immune response.
OVX836 will be evaluated against two pandemic influenza A-strains in preclinical models: the once pandemic but now seasonal, pH1N1, and the highly pathogenic variant with pandemic potential, H5N1.
Osivax’s oligoDOM® technology enables the design and production of a recombinant version of the NP, which self-assembles into a nanoparticle, thus triggering powerful T- and B-cell immune responses.
OVX836 has been tested in 5 clinical trials with 1200 participants, showing promising safety, immunogenicity, and efficacy read-outs.
“Receiving this grant from the NIAID will support our progress in developing OVX836 to provide broad-spectrum protection against influenza, which remains a perennial pandemic threat,” commented Alexandre Le Vert, Co-Founder and CEO of Osivax, in a December 5, 2023 press release.
“We believe that by generating these additional data against pandemic influenza strains, we will be able to bolster the positive results generated by OVX836 against seasonal strains, placing us on a strategic path toward future regulatory approval.”
Osivax also recently published the results of a Phase 2a dose-optimization study (OVX836-003) in The Lancet Infectious Diseases, showing efficacy in humans against seasonal strains.