Search API
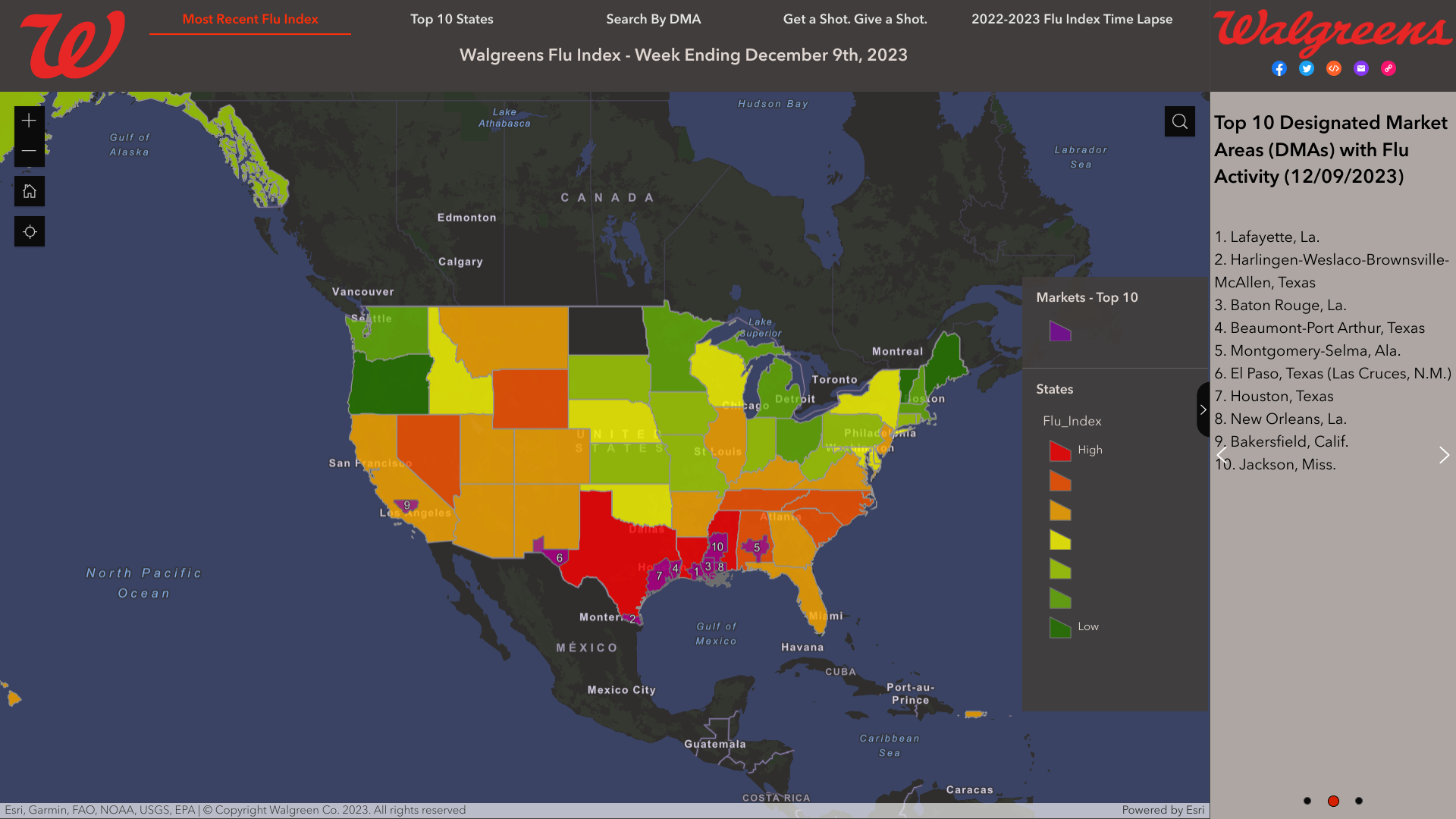
As national influenza rates increased post-Thanksgiving, according to the Walgreens Flu Index®'s latest report, the state of Texas is leading the nation in influenza activity.
As of December 9, 2023, the Index identified the leading cities using retail prescription data for antiviral medications used to treat influenza across Walgreens locations nationwide.
- Lafayette, La.
- Harlingen-Weslaco-Brownsville-McAllen, Texas
- Baton Rouge, La.
- Beaumont-Port Arthur, Texas
- Montgomery-Selma, Ala.
- El Paso, Texas (Las Cruces, N.M.)
- Houston, Texas
Anita Patel, PharmD, Vice President of Pharmacy Service Development at Walgreens, commented in a press release on December 7, 2023, ".... practicing good respiratory etiquette are all important steps to protect yourself and your loved ones this winter, especially if you are feeling sick or planning to travel and gather for the holidays."
Testing is the best way to know for sure if you have a specific respiratory virus so you can take appropriate precautions and get the proper relief or treatment immediately.
Once you know your test results, your pharmacist can help you determine the best next steps and get the appropriate treatment as soon as possible, whether that's a prescription medicine or over-the-counter essentials to manage your symptoms, says Walgreens.
Furthermore, the U.S. CDC recommends getting an annual flu shot before gathering with friends and family this holiday season.
As of December 2, 2023, over 152 million flu vaccines (nasal, cell-based, egg-based) have been distributed in the U.S. and are available at most clinics and pharmacies.
The Walgreens Flu Index is not intended to illustrate levels or severity of flu activity but rather to illustrate which populations are experiencing the highest incidence of influenza.
Icosavax, Inc. today announced it had entered into a definitive agreement under which AstraZeneca would purchase the company Phase 2 study of IVX-A12, a combination virus-like particle (VLP) vaccine candidate targeting both respiratory syncytial virus (RSV) and human metapneumovirus (hMPV).
There are currently no treatments or preventative therapies for hMPV. Adults with hMPV infection may have viral pneumonia, worsening asthma, or COPD symptoms. And there are no combination vaccines for RSV.
Announced on December 11, 2023, Iskra Reic, Executive Vice President, Vaccines & Immune Therapies, AstraZeneca, commented in a press release, "This VLP vaccine technology has the potential to transform prevention against severe infectious diseases, including RSV and hMPV."
"With the addition of Icosavax's Phase III-ready lead asset to our late-stage pipeline, we will have a differentiated, advanced investigational vaccine and a platform for further development of combination vaccines against respiratory viruses."
"This aligns with our strategy to deliver a portfolio of therapies to address high unmet needs in infectious diseases and our ambition to protect the most vulnerable patients who have a high risk of severe outcomes."
Separately, Icosavax announced positive topline interim results from its Phase 2 clinical trial of IVX-A12 against RSV and hMPV in older adults.
IVX-A12 induced robust immune responses against both RSV and hMPV at Day 28 across both formulations with and without adjuvant.
"We're delighted to announce positive topline interim data from our Phase 2 trial of IVX-A12, our potential first-in-class combination vaccine candidate against RSV and hMPV," said Adam Simpson, Chief Executive Officer of Icosavax, in a press release.
"We believe that IVX-A12 has the potential to address a significant unmet need and, as the furthest advanced RSV and hMPV combination vaccine in the clinic, to build on an emerging, large market opportunity."
The ongoing Phase 2 clinical trial of IVX-A12 is a randomized, observer-blinded, placebo-controlled, multicenter trial designed to evaluate the safety and immunogenicity of a single dose of RSV and hMPV combination VLP vaccine IVX-A12, with and without CSL Seqirus' proprietary adjuvant MF59®.
Regarding the proposed acquisition, the upfront cash portion of the consideration represents an equity value of approximately $838 million, a 43% premium over Icosavax's closing market price on December 11, 2023, and a 73% premium to Icosavax's volume-weighted average price for the preceding 60 trading days.
Combined, the upfront and maximum potential contingent value payments represent, if achieved, an equity value of approximately $1.1 billion, a 91% premium over Icosavax's closing market price on December 11, 2023, and a 130% premium to Icosavax's volume-weighted average price for the preceding 60 trading days.

Alzamend Neuro, Inc. today announced receipt of a "Study May Proceed" letter from the U.S. Food and Drug Administration ("FDA") for the initiation of study AL001-PTSD01, a Phase IIA clinical study of AL001 for the treatment of patients with post-traumatic stress disorder ("PTSD").
AL001 is a novel lithium-delivery system that can potentially deliver the benefits of marketed lithium salts while mitigating or avoiding currently experienced toxicities associated with lithium.
"Although lithium does not have an FDA-approved indication for PTSD, it has been prescribed off-label for this purpose for decades," said Stephan Jackman, Chief Executive Officer of Alzamend, in a press release on December 11, 2023.
"If we can develop a next-generation lithium product (AL001) that would not routinely require therapeutic drug monitoring, it would constitute a major improvement over current lithium-based treatments and positively impact the 9 million Americans afflicted with PTSD."
"We are advancing the process and expect that the first patient will be dosed in the first quarter of 2024."
AL001 is designed to favorably distribute lithium in the brain resulting in lower exposure to other body organs and an improved safety profile compared to currently marketed lithium salts.
This can serve to mitigate or obviate the disadvantageously low ceiling for toxicity of marketed lithium salts that have limited their usefulness to patients and prescribers.
PTSD is a mental and behavioral disorder that can develop because of exposure to a traumatic event, such as sexual assault, warfare, traffic collisions, child abuse, domestic violence, or other threats to a person's life.
According to the U.S. NIH, about 3.6% of adults in the U.S. have PTSD in a given year, and 9% of people develop it at some point in their life. Worldwide, rates for PTSG in a given year are between 0.5% and 1% of the population.

Merck and Moderna, Inc. today announced the initiation of a pivotal Phase 3 randomized clinical trial (INTerpath-002) evaluating V940 (mRNA-4157), an investigational individualized neoantigen therapy, in combination with KEYTRUDA®, Merck’s anti-PD-1 therapy, as adjuvant treatment in patients with completely resected (R0) Stage II, IIIA or IIIB (with nodal involvement) non-small cell lung cancer.
Global recruitment of the INTerpath-002 has begun, and the first patients enrolled in Australia.
“As lung cancer is the leading cause of cancer death worldwide, there is a need for continued scientific advancements to help fight this disease at earlier stages when patients have the best chance for better outcomes,” said Dr. Marjorie Green, senior vice president and head of late-stage oncology, global clinical development, Merck Research Laboratories, in a press release on December 11, 2023.
“By combining KEYTRUDA with V940 (mRNA-4157), a promising new modality, we are researching innovative new approaches for earlier stage non-small cell lung cancer.”
As previously announced, in addition to INTerpath-002, the combination of V940 (mRNA-4157) plus KEYTRUDA is being investigated in INTerpath-001, a global, randomized, double-blind, placebo- and active-comparator-controlled Phase 3 trial evaluating patients with resected high-risk (Stage IIB-IV) melanoma.
INTerpath-001 is actively screening in 14 countries, representing 38 sites.
The companies confirmed they plan to expand the comprehensive clinical development program for V940 (mRNA-4157) to additional tumor types.

The U.S. Centers for Disease Control and Prevention (CDC) continues to publish Trave Health Notices regarding diphtheria outbreaks in various countries in 2023.
On December 7, 2023, the CDC posted a Level 2 - Practice Enhanced Precautions notice regarding an outbreak of diphtheria in several districts in Guinea, which is located in western Africa.
Diphtheria is a severe infection caused by strains of Corynebacterium diphtheriae bacteria that make a toxin. The toxin can cause people to get very sick. Diphtheria bacteria spread from person to person through respiratory droplets, like from coughing or sneezing.
People can also get sick from touching open sores or ulcers of people ill with diphtheria, according to the CDC.
Diphtheria is a vaccine-preventable disease.
Unfortunately, an estimated 16% of children worldwide had no or incomplete vaccination coverage.
The U.S. CDC says most travelers visiting outbreak areas should receive an age-appropriate dose of diphtheria toxoid-containing vaccine if they are not fully vaccinated or have not received a booster dose within five years before departure.
There are 11 vaccines available for use to help protect against diphtheria in 2023. Diphtheria and other travel vaccines are offered at many clinics and pharmacies in the U.S.

The Africa Centres for Disease Control and Prevention (Africa CDC) recently welcomed the announcement from The Global Vaccine Alliance (GAVI) Board for the establishment of the African Vaccine Manufacturing Accelerator (AVMA).
The AVMA is a financing mechanism to create a sustainable vaccine manufacturing industry in Africa. It will make up to $1 billion in funds available to support vaccine manufacturing in Africa.
Dr. Jean Kaseya, Africa CDC Director General, commented in a press release on December 8, 2023, "Today is a significant moment for Africa. The targeted USD 1 Billion from GAVI to African Manufacturers is a game changer for the continent and advances our efforts towards vaccine self-reliance."
"The African Union has set a target for the continent to produce 60% of the vaccines needed by 2040, and the AVMA is indeed an accelerator towards that ambition."
"GAVI has been an incredible partner in this; we will continue to advance together on this journey of self-reliance. Together, we are united with a mission for vaccine equity."
The launch of AVMA is an important message from our partners that Africa will no longer be solely a recipient of vaccines but an active member and contributor to the global vaccine ecosystem.
The collaboration has seen several vaccine manufacturing projects taking shape, and others are in the works to guarantee self-reliance in Africa should any health emergency or outbreak hit the continent.
In addition, Africa CDC remains committed to collaborating with all partners and stakeholders in the vaccine ecosystem to facilitate the full operationalization of AVMA and expedite the attainment of health security, as envisaged in Agenda 2063.
The Coalition for Epidemic Preparedness Innovations (CEPI) recently announced it had partnered with Jurata Thin Film, Inc. to advance development of thermostable under-the-tongue vaccine films as a needle-free vaccine delivery platform.
On December 5, 2023, CEPI confirmed that it will provide up to an initial $1.2 million to support Jurata's proprietary innovative formulation platform, which, if shown to be successful, could help expand access to vaccines in underserved regions and advance the global response to future emerging infectious disease outbreaks.
CEPI's initial funding will support optimizing the composition and process of creating thin films and preclinical studies.
Under the agreement with CEPI, Jurata will create vaccine films to remain stable at 2-8 degrees, 25 degrees, and 40 degrees.
Jurata will optimise the composition of the films by testing various buffers, pH, stabilizers, sugars, salts, and different drying parameters and assessing how this affects vaccine stability and delivery.
Jurata aims to improve vaccine accessibility by stabilizing the 3D structure of mRNA-containing lipid nanoparticle vaccine materials, provided by Quantoom Biosciences, part of Univercells, into a thin thermostable film, thereby removing frozen storage needs.
The vaccine films are also lightweight and compact, simplifying the transportation process and potentially allowing for more doses to be shipped at any one time compared to current needle-and-syringe distribution.
Dr. Irnela Bajrovic, Chief Scientific Officer, Jurata, commented in a press release, "Our stabilising formulations have the potential to facilitate global access to mRNA vaccines, and our thin film delivery platform could make vaccine administration far easier than needle-and-syringe injections."
"We are grateful to CEPI for supporting our innovative technology and look forward to working with Quantoom to show the breadth of mRNA vaccines our technology can stabilize and deliver."
This is the fourth partner to be announced as part of CEPI's Call for Proposals for thermostable vaccine manufacturing innovations, announced in January 2022.
Thermostable vaccines are also identified as a preferred vaccine characteristic by the World Health Organization.

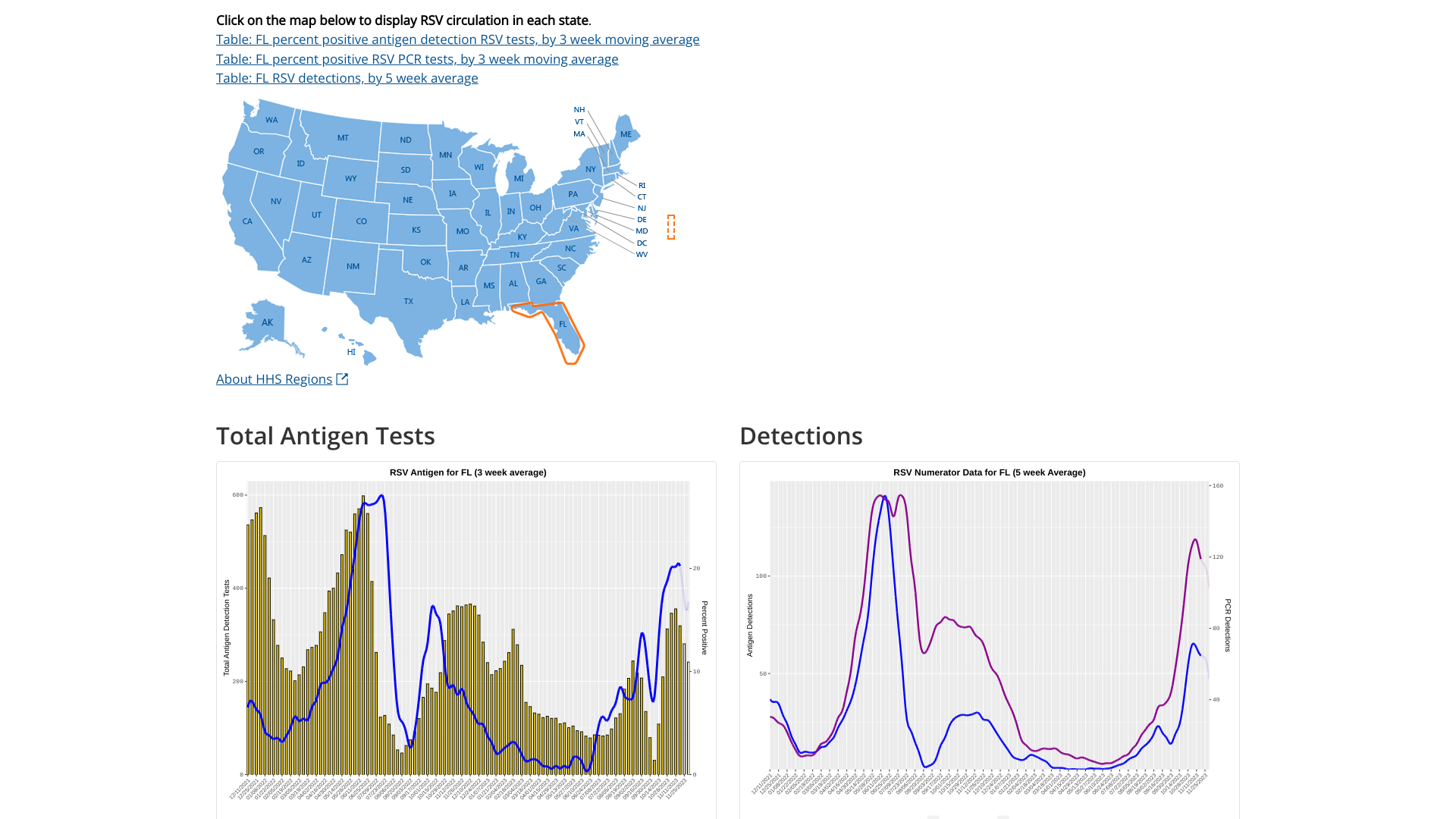
Since most respiratory syncytial virus (RSV) activity in the United States is initially recorded in the state of Florida, watching for the bending of the curve offers insights regarding future cases.
As of December 8, 2023, the U.S. Centers for Disease Control and Prevention (CDC) updated its RSV detection graphs to display the 5-week moving average. RSV infections typically occur during late fall, winter, and early spring.
There are variations in the timing of RSV outbreaks between regions and communities in the same region.
For Florida, the two charts indicate RSV's peak was in late November 2023.
Separately, the Florida Department of Health reported that as of December 2, 2023, RSV activity was decreasing in hospital admissions, positivity tests, and ER visits.
But, there was an RSV outbreak in Pinellas County.
From a prevention perspective, both RSV vaccines are available at most clinics and pharmacies in Florida. However, the CDC recently confirmed the percentage of seniors receiving an RSV vaccine was just 15.9%.
Unfortunately, the RSV antibody passive immunization for infants, Beyfortus™, remains in limited supply.
Virtually all children get an RSV infection by the time they are two years old. Most of the time, RSV will cause a mild, cold-like illness. RSV antibodies can help protect children from severe disease from an RSV infection.
RSV can be dangerous for infants and young children. Each year, thousands are hospitalized due to RSV infection, says the CDC.