Search API
Invivyd, Inc. today announced positive initial results from the ongoing CANOPY Phase 3 pivotal clinical trial of VYD222, a broadly neutralizing, half-life extended monoclonal antibody (mAb) candidate for the prevention of symptomatic COVID-19.
Results showed that the safety and tolerability profile of VYD222 remains favorable, with no study drug-related serious adverse events reported to date.
The company also reported on December 14, 2023, that in vitro pseudovirus testing shows VYD222 has potency against various SARS-CoV-2 coronavirus variants currently circulating, such as HV.1, BA.2.86, XBB.1.5.10/EG.5, and HK.3.
Importantly, VYD222 continues to show neutralizing activity against variants with the F456L mutation found in most variants in the U.S.
Currently, no mAb is authorized or approved in the U.S. to prevent symptomatic COVID-19.
"We are pleased to share positive initial topline results from CANOPY, which bolster our belief that VYD222 holds the potential to provide vulnerable people, particularly the immunocompromised (IC), with meaningful protection from COVID-19," said Dave Hering, Chief Executive Officer of Invivyd, in a press release.
"VYD222 produced high serum virus neutralizing antibody (sVNA) titer levels against XBB.1.5 in the IC cohort, essentially replicating the titer levels observed in our Phase 1 clinical trial of VYD222 in healthy volunteers."
"We are also encouraged by the potential early signal of strong clinical protection from symptomatic COVID-19 in the CANOPY clinical trial to date, which would be expected given the high VYD222 sVNA titer levels and dose selected."
"We look forward to continued engagement with the FDA on these promising results, and we intend to submit a request for Emergency Use Authorization as soon as practicable."
Globally, there are millions of immunocompromised people, with more than 9 million in the U.S. alone who may not adequately respond to COVID-19 vaccination.

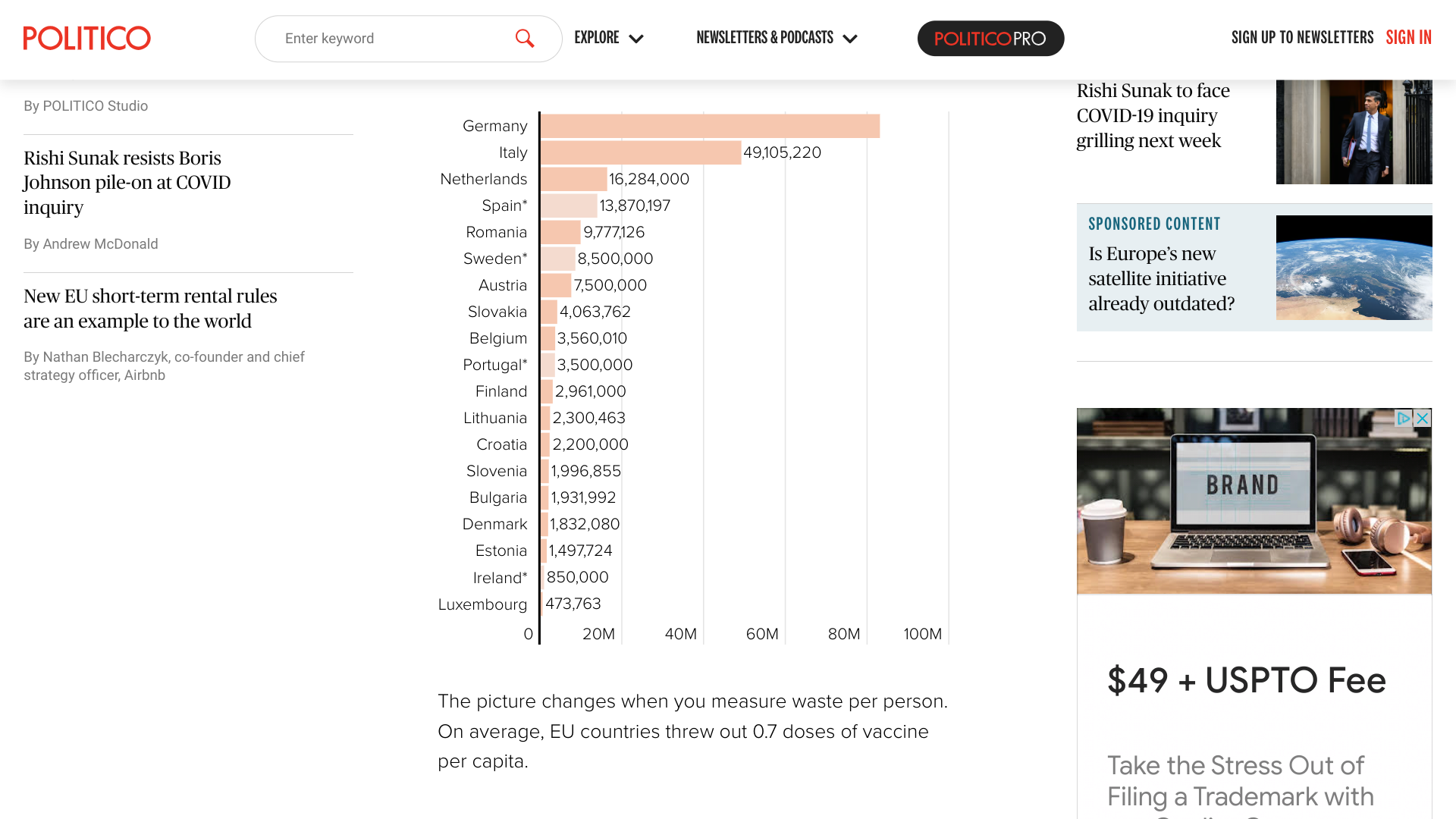
POLITICO reported today at least 215 million COVID-19 vaccines purchased by European Union (EU) countries have been recently discarded at an estimated cost of $4.3 billion.
Since COVID-19 vaccines became available in late 2020, EU countries have collectively taken delivery of 1.5 billion doses.
POLITICO's December 18, 2023 announcement is based on data from 19 EU countries. Germany, for example, provided its vaccine waste figures to POLITICO in June 2023.
As 2024 approaches, purchases for updated COVID-19 vaccines continue.
On December 13, 2023, the World Health Organization (WHO) announced that given the current SARS-CoV-2 betacoronavirus evolution and the breadth in immune responses demonstrated by monovalent XBB.1.5 vaccines, the Technical Advisory Group on COVID-19 Vaccine Composition advises retaining the current COVID-19 vaccine antigen composition (monovalent XBB.1.5) into 2024.
There are 12 COVID-19 vaccines currently Listed by the WHO.
In the U.S., only three vaccines are available.
As of December 2, 2023, only 7.7% of children were up to date with the 2023-24 COVID-19 vaccine, as reported by the U.S. CDC.
And 17.2% of adults reported receiving an updated 2023-24 COVID-19 vaccine since September 14, 2023.
In the U.S., 2023-24 COVID-19 vaccine coverage data using interactive maps, trend lines, bar charts, and tables are available at this CDC link.
The U.S. National Center for Health Statistics (NCHS) Mortality Surveillance mortality surveillance data indicates that 0.2% of deaths during the week ending December 9, 2023 (Week 49) were due to influenza.
This NCHS percentage remained stable compared to Week 48.
Unfortunately, the U.S. CDC reported two additional influenza-associated pediatric deaths last week.
As of December 14, 2023, a total of 14 influenza-associated pediatric deaths, vaccination status not reported, have occurred during the 2023-2024 flu season.
The CDC reported seasonal influenza activity remains elevated in most parts of the country and recommends that most people over six months get an annual flu shot.
Furthermore, based on when you get a flu shot, your health condition, and where you live, a second vaccination may be appropriate based on a conversation with a doctor, nurse, or pharmacist.
During this flu season, over 152 million nasal, egg-based, and cell-based influenza vaccines were distributed in the U.S. These vaccines remain available at most pharmacies.

According to several health agencies, the global risk assessment related to dengue virus outbreaks increased in late December 2023.
The European Centre for Disease Prevention and Control reported that as of November 2023, over 4.5 million cases and over 4,000 dengue-related deaths have been reported from 80 countries/territories globally.
For example, the U.S. CDC reissued Level 1 - Practice Usual Precautions, Travel Health Notices on December 14, 2023, for countries located in Africa, Asia, the Americas, the Middle East, and the Pacific Islands.
In the Americas, the Pan American Health Organization (PAHO) confirmed that 2023 is the year with the highest historical record of dengue cases, registering more than 4.1 million new infections, exceeding 2019, when about 3.1 million cases were reported.
Several factors are associated with the increasing risk of dengue epidemics, such as the changing range in elevation of Aedes aegypti mosquitoes, especially in previously dengue native areas, stated the PAHO on December 12, 2023.
Because mosquito bites spread dengue, all travelers to these areas, including southeast Florida and Puerto Rico, are at risk.
Since dengue can become severe within a few hours, usually requiring hospitalization, prevention options are essential.
In addition to avoiding mosquito bites, dengue is a vaccine-preventable disease.
As of late 2023, two approved dengue vaccines and several vaccine candidates are in development. These vaccines have limited availability, based on which country you are located in.

A recent study published by The New England Journal of Medicine confirmed the benefits of getting a quadrivalent recombinant influenza vaccine this flu season.
Although numerous clinical studies have shown that high-dose influenza vaccines (Flublok®) offer benefits over egg-based flu shots, “Data are needed on the relative effectiveness of recombinant vaccines as compared with standard-dose vaccines against influenza-related outcomes in adults under the age of 65 years,” wrote these researchers on December 14, 2023.
The Sanofi-funded study population included 1,630,328 vaccinees between 18 and 64 years old.
During this study period, 1,386 cases of PCR-confirmed influenza were diagnosed in the recombinant-vaccine group and 2,435 cases in the standard-dose group.
Among the participants who were 50 to 64 years of age, 2.00 cases per 1000 tested positive for influenza in the recombinant-vaccine group compared to 2.34 cases per 1000 in the standard-dose group.
This data indicates a relative vaccine effectiveness of 15.3%; 95% confidence interval [CI], 5.9 to 23.8; P=0.002).
However, the recombinant vaccine was not significantly more protective against influenza-related hospitalization than were the standard-dose vaccines.
In summary, the high-dose recombinant vaccine conferred more protection against PCR-confirmed influenza than an egg-based standard-dose vaccine among adults between 50 and 64 years old.
Flublok is made by Sanofi and is licensed by the U.S. Food and Drug Administration and the European Medicines Agency as Supemtek.

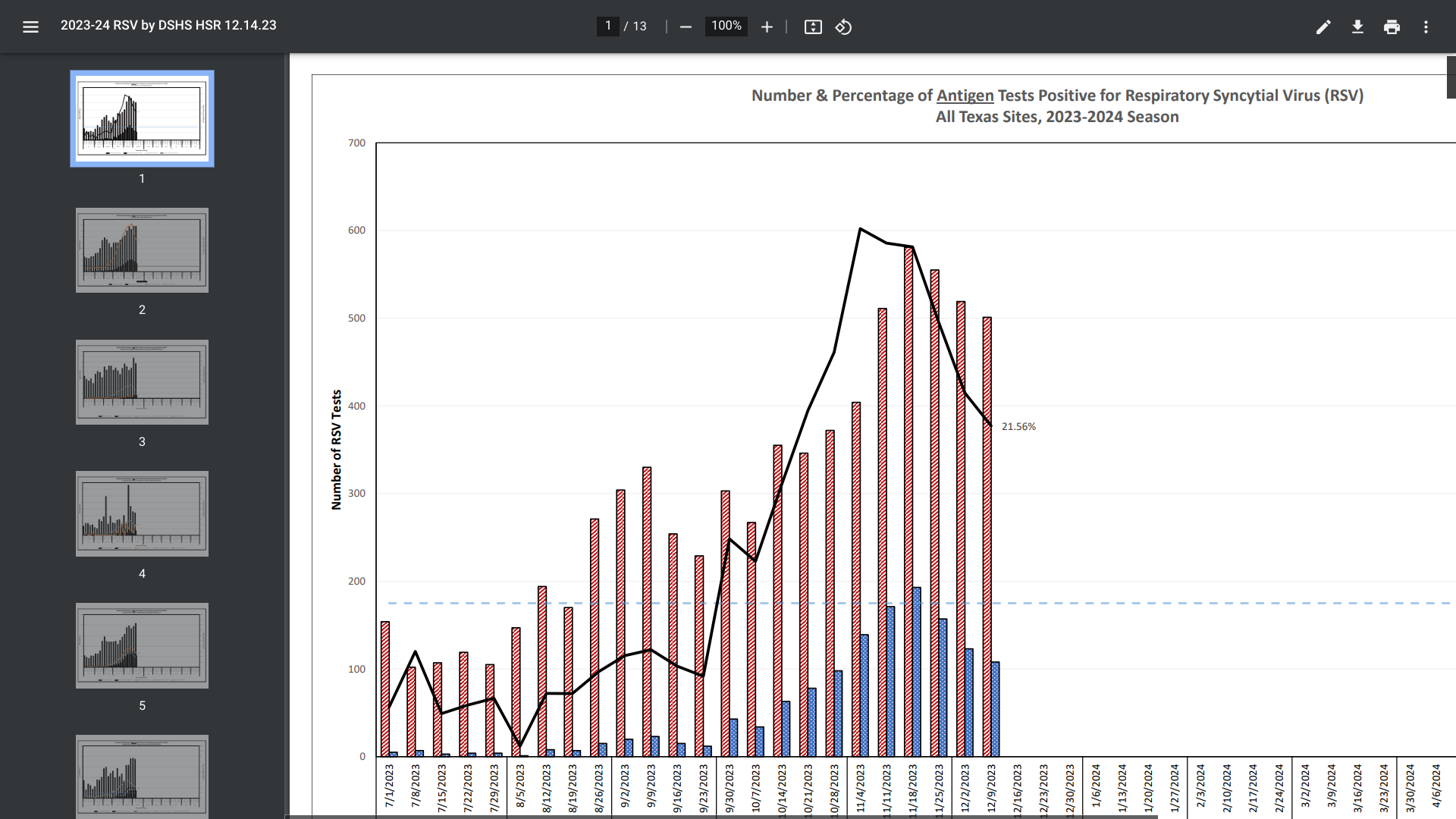
According to recent data published by the Texas Department of State Health Services (DSHS) and the U.S. CDC, Respiratory Syncytial Virus (RSV) trend data indicates the 2023-2024 season has begun to end.
RSV activity typically begins to increase in Texas in September or October and peaks in December or January.
As of December 14, 2023, DSHS reported the number (500) and percentage (21.56%) of Antigen Tests Positive for RSV had decreased from a peak during the middle of November 2023.
However, there is currently insufficient data to properly present trends for the Upper Rio Grande/El Paso region.
RSV can spread when a person with the virus coughs or sneezes into the air. School-aged children who are infected with RSV and have a mild upper respiratory tract infection often introduce RSV into the home, says DSHS.
For infants and young children, a one-time injectable monoclonal antibody called Beyfortus™ (Nirsevimab-alip) is available to prevent severe RSV illness. Beyfortus cannot prevent infection with RSV or help cure or treat children already suffering from serious RSV disease.
For older adults, there are two approved RSV vaccines available in 2023. About 17% of qualifying adults have received an RSV vaccine this year.
Sanofi and AstraZeneca recently announced they continue to engage with government officials, healthcare providers, and others in the United States regarding the supply of Beyfortus™ (nirsevimab-alip), a respiratory syncytial virus (RSV) monoclonal antibody therapy.
On December 14, 2023, the companies wrote, Since we launched in September 2023, hundreds of thousands of infants in the U.S. have received Beyfortus: 50 mg or 100mg Injection.
This is the first season in which passive immunization for babies against RSV has been provided broadly.
By the end of January 2024, a total of 1.4 million babies will be offered protection against RSV, a 27% increase over the initial supply forecast for the 2023-2024 RSV season in the U.S.
Beyfortus is a prescription injectable medicine used to help prevent a serious lung disease caused by RSV in babies under one year of age born during or entering their first RSV season and for children up to 24 months of age who remain at risk of severe RSV disease through their second RSV season.
It is a recombinant human immunoglobulin G1 kappa (IgG1ĸ) long-acting mAbs that binds to the prefusion conformation of the RSV F protein.
The most common side effects of Beyfortus include rash and pain, swelling, or hardness at the site of your child’s injection.
On October 23, 2023, the U.S. CDC published Interim Recommendations to Protect Infants from RSV (CDCHAN-00499) during the 2023–2024 RSV season. RSV antibody therapy was initially authorized in the U.S. in 1998.

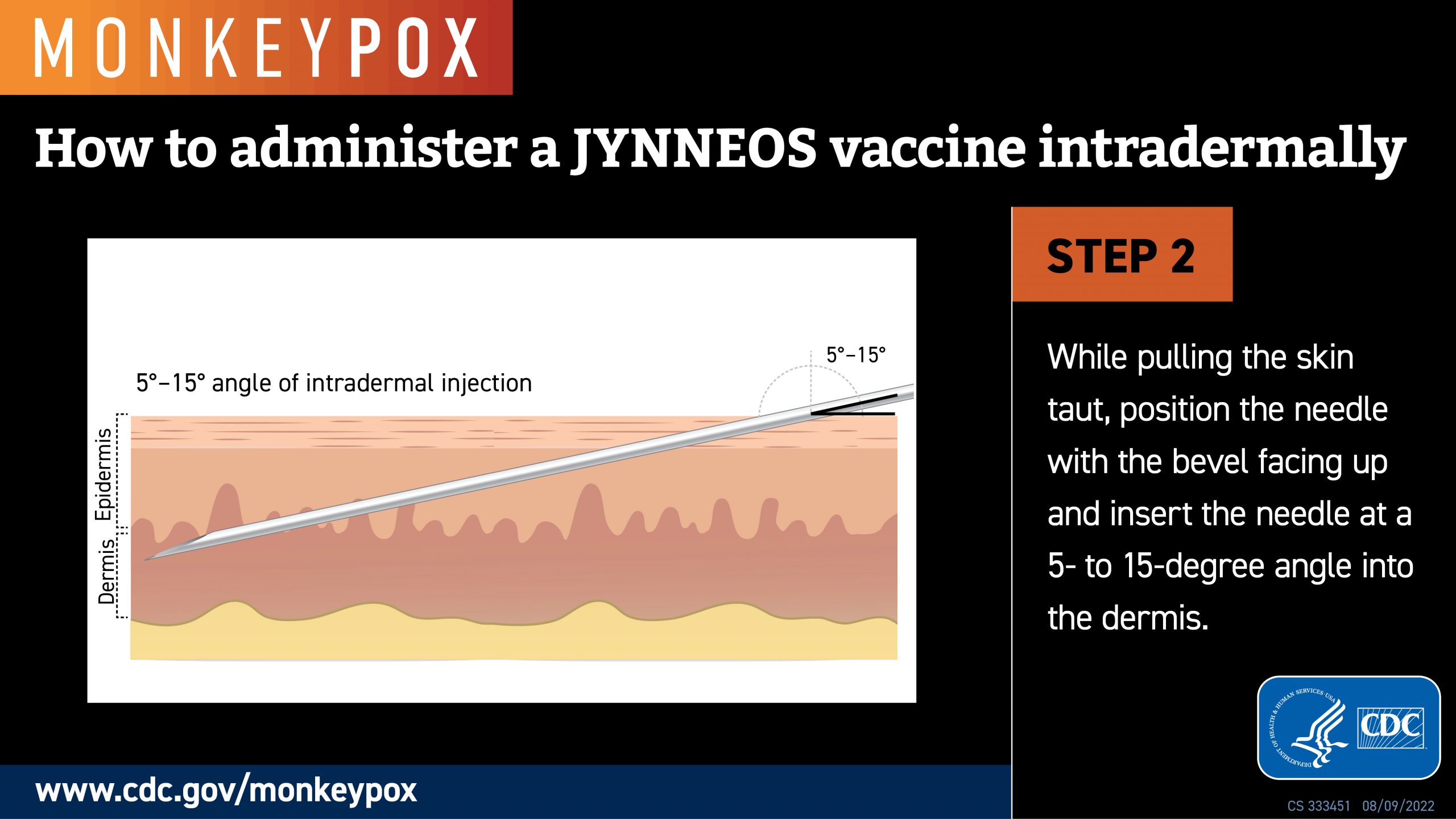
Led by researchers at NYU Grossman School of Medicine, a recent study showed no significant difference in the strength of the immune response (IgG antibodies) between most of those who received their vaccine injections in small doses between layers of the skin.
In some cases, the IgG antibodies were detected more than six months after the second and final JYNNEOS® vaccination.
Because of limited space between skin layers, intradermal injections can only accommodate small doses, while larger doses generally require subcutaneous injections.
The smaller doses, about one-fifth of the usual full dose and spread out by as long as three months, were authorized by the U.S. FDA and CDC in August 2022.
About 155,000 New Yorkers have been vaccinated, mainly using smaller doses.
"Our study shows that smaller vaccine doses of mpox vaccine administered in two doses spread out over weeks to months were similar to the full (subcutaneous) FDA-approved dose," said study co-lead investigator and infectious disease specialist Angelica Cifuentes Kottkamp, MD, in a press release on December 14, 2023,
"Implementing the smaller dose was a good emergency measure in the face of immediate shortages of the vaccine," said Dr. Kottkamp, an assistant professor in the Department of Medicine at NYU Langone Health.
Additionally, the New England Journal of Medicine published a Correspondence that revealed people fully vaccinated with two smaller JYNNEOS doses had an immune response four times stronger than those who did not complete the vaccination series and had only one dose.
This study's finding is significant since The Lancet Infectious Diseases reported on December 7, 2023, that 12% of JYNNEOS vaccinated individuals were non-antibody responders.
Bavarian Nordic codeveloped JYNNEOS with the U.S. Government to ensure adult populations, including people with weakened immune systems, could be protected from smallpox.
On February 22, 2023, the U.S. CDC issued Interim Clinical Considerations confirming that mpox vaccination should continue to be offered to people with the highest potential for exposure to mpox. The U.K. and Europe have issued similar notices in 2023.
Novavax Inc. recently announced its updated protein-based COVID-19 vaccine is now the only COVID-19 vaccine option available in the Republic of Poland.
Doses were distributed by the appropriate Polish authorities in 2023 and made available for this season's vaccination campaign.
Novavax's vaccine is available in Polato to prevent COVID-19 in individuals aged 12 and older.
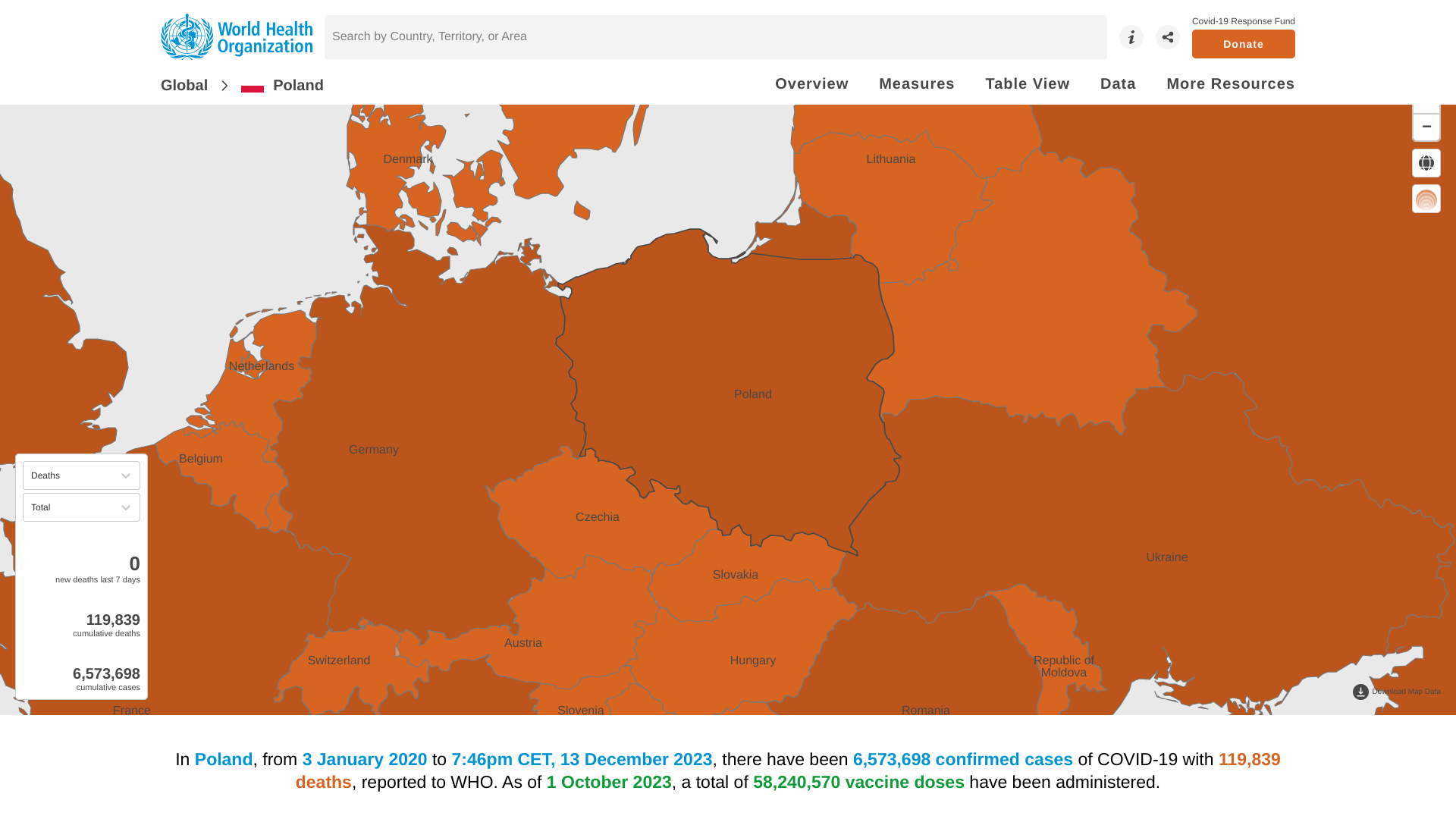
In Poland, from January 2020 to early December 2023, there have been 6,573,698 confirmed cases of COVID-19, with 119,839 related deaths.
As of October 2023, a total of 58,240,570 doses of various COVID-19 vaccines had been administered in Poland.
We are pleased that our updated vaccine is available in time for the upcoming Christmas and winter holidays, wrote the company on December 11, 2023. We are honored to support the Polish government and the country's healthcare workers in helping to protect Polish citizens and their loved ones against COVID-19.
Recent non-clinical data showed that Novavax's updated COVID-19 vaccine induced functional immune responses against XBB.1.5, XBB.1.16 and XBB.2.3 variants. Additional non-clinical data demonstrated that Novavax's vaccine-induced neutralizing antibody responses to subvariants BA.2.86, EG.5.1, FL.1.5.1, and XBB.1.16.6 as well as CD4+ polyfunctional cellular (T-cell) responses against EG.5.1 and XBB.1.16.6.
These data indicate that Novavax's vaccine can stimulate both arms of the immune system and may induce a broad response against currently circulating variants.
As of December 14, 2023, Sweden and Italy have authorized Novavx's updated vaccine. Throughout the recent pandemic, Novavax COVID-19 Vaccines have been authorized in Europe, the U.S., the U.K., and numerous other countries under various brands.
The U.S. government today announced that Sanofi and AstraZeneca will make available 230,000 additional doses of a new passive immunization that prevents lower respiratory tract infections in infants caused by the respiratory syncytial virus (RSV).
Announcement on December 14, 2023, the additional doses of Beyfortus™ (Nirsevimab-alip) are scheduled for delivery in January 2024.
Beyfortus, an extended half-life monoclonal antibody, was approved by the U.S. FDA in July 2023.
This new allocation is in addition to the 77,000 Beyfortus doses released to the U.S. in November 2023.
Sanofi had previously confirmed that demand for this product, especially for the 100 mg doses, had been higher than anticipated.
In clinical trials, a single injection reduced the chances of severe RSV infection by 74.5%.
RSV disease can be prevented either by giving antibody products to infants and young children or by giving their mothers RSV vaccine during pregnancy, says the U.S. CDC.
According to a Johns Hopkins news article published in July 2023, monoclonal antibodies work by providing immediate and short-term protection, whereas vaccines “boost your immunity in the future.
David Dowdy, MD, professor in Epidemiology, explained that your immune system can’t “learn” anything from an antibody. The drug is “basically to protect during a time of life when people are most vulnerable.”
The protection that Beyfortus provides is called “passive immunity” because it does not come from the person’s immune system.
