Search API
Since Zika outbreaks peaked in 2017, the circulation of this mosquito-borne virus has been confirmed in 89 countries worldwide. Although incidence levels remain low, sporadic increases in Zika cases have been observed in some countries in the Region of the Americas.
The Zika virus is a flavivirus transmitted by mosquitoes of the Aedes genus, which are common in the Americas.
"Most infections with this virus are asymptomatic or mild, making their detection by healthcare systems quite challenging," said María Van Kerkhove, Head of the Emerging Diseases and Zoonoses Unit at the World Health Organization (WHO), in a September 2023 press release.
However, the Pan American Health Organization confirmed that the Federative Republic of Brazi confirmed 33,386 Zika cases last year.
And with climate change impacting the range of disease-carrying mosquitos, several countries in Central America reported Zika outbreaks as of week #52.
- Belize - 281
- Guatemala - 112
- El Salvador - 110
- Mexico - 29
But this trend has yet to progress north of Mexico and the Rio Grande River, as the state of Texas reported (0) Zika cases in 2023.
With regard to complications from this disease, WHO says many people infected with the Zika virus won't have symptoms or will only have mild symptoms.
Unfortunately, pregnant women are particularly susceptible to its effects since it can lead to congenital malformations, such as microcephaly, as well as an increased likelihood of preterm births or spontaneous abortions.
As of January 11, 2024, there are no U.S. FDA-approved Zika vaccines, but several vaccine candidates are conducting clinical research.
According to a letter by a France-based penicillin producer, the U.S. Food and Drug Administration (FDA) has authorized the importation of an injectable drug to treat syphilis.
A letter dated November 21, 2023, Paris-based from Laboratoires Delbert stated: Temporary Importation of Extencilline (benzathine benzylpenicillin) Powder and diluent for reconstitution for injection, 1,200,000 units and 2,400,000 units with Foreign, non-U.S. Labeling to Address Supply Shortage.
Extencilline will be available in the U.S. only by prescription in 2024.
The FDA's website confirmed this authorization on January 10, 2024.
This change in FDA policy is related to the current penicillin supplier having an available disruption.
On June 12, 2023, Pfizer notified the FDA that ll Bicillin ® C-R (penicillin G benzathine and penicillin G procaine injectable suspension) are estimated to deplete in Q3 2023 and Bicillin® L-A (penicillin G benzathine injectable suspension) Pediatric (600,000 units/mL) Prefilled Syringes are estimated to deplete by the end of Q2 2023. Furthermore, these penicillin presentations will not be available until further notice.
Key differences between Bicillin® L-A and Extencilline are available at this link.
Neither penicillin injectable drug is a U.S. FDA-approved syphilis vaccine.
The continued prevalence of Syphilis and Congenital Syphilis in the United States highlights the need for a syphilis preventive vaccine, says the World Health Organization.

Emergent BioSolutions Inc. today announced that it has secured an indefinite-delivery, indefinite-quantity (IDIQ) procurement contract with a maximum value of up to $235.8 million to supply BioThrax® (Anthrax Vaccine Adsorbed) for use by all branches of the United States military as Pre-Exposure Prophylaxis (PrEP) for anthrax disease.
The new contract with the U.S. Department of Defense (DoD), led by the Joint Program Executive Office for Chemical, Biological, Radiological, and Nuclear Defense, is comprised of a five-year base agreement ending on September 30, 2028, and an additional five-year option that would extend the contract to September 30, 2033.
Under the initial five-year IDIQ contract, there is a guaranteed purchase minimum of $20.1 million, with future orders estimated at least $20 million each following year.
"As a part of our mission to protect and enhance lives, Emergent is proud to continue supporting and preparing our nation's service members who have a high risk of exposure to anthrax bacteria by supplying BioThrax vaccine," said Paul Williams, senior vice president, products head at Emergent, in a press release on January 11, 2024.
Emergent's BioThrax vaccine is indicated for active immunization to prevent disease caused by Bacillus anthracis in persons 18 through 65 years of age.
BioThrax is also approved for pre-exposure prophylaxis of disease in persons at high risk of exposure.
Furthermore, BioThrax is approved for post-exposure prophylaxis of disease following suspected or confirmed Bacillus anthracis exposure when administered in conjunction with recommended antibacterial drugs.
Biothrax's initial approval in the U.S. was issued in 1970. The U.S. FDA updated STN: BL 103821 in July 2023.

The Oxford Vaccine Group today announced it is leading the first-in-human phase 1 clinical trial of the ChAdOx1 NipahB vaccine candidate.
Funded by the Coalition for Epidemic Preparedness Innovations (CEPI), this vaccine candidate is being developed by researchers at the University of Oxford's Pandemic Sciences Institute.
Fifty-one adults aged 18 to 55 will participate in the trial, which is expected to last 18 months (March 2025), with additional trials expected to follow in a Nipah-affected country, such as Singapore, Malaysia, Bangladesh, or India.
The primary contact of the study (ISRCTN87634044) is Miss Ella Morey, [email protected].
This new study is essential as there are no approved Nipah vaccines to protect people from this disease, which is fatal in around 75% of cases.
Professor Brian Angus, the trial's Principal Investigator and Professor of Infectious Diseases at the Centre for Clinical Tropical Medicine and Global Health commented in a press release on January 11, 2024, "Nipah virus was first identified in 1998, and yet 25 years on the global health community still has no approved vaccines or treatments for this devastating disease."
"Due to the high mortality rate and the nature of Nipah virus transmission, the disease is identified as a priority pandemic pathogen. This vaccine trial is an important milestone in identifying a solution to prevent local outbreaks while helping the world prepare for a future global pandemic."
The World Health Organization (WHO) recognizes Nipah as a priority disease requiring urgent research and belongs to the same family of paramyxoviruses as more well-known pathogens like measles.
According to the WHO, fruit bats carry the disease and may also be transmitted by contact with infected animals (pigs) or from person to person via close contact.
Initially announced in 2023, the Republic of Cameroon intends to launch a malaria vaccine campaign in late January 2024. Cameroon took delivery of 331,200 doses of malaria vaccine in November last year.
According to Manaouda Malachie, Cameroon's minister of public health, this vaccination effort is part of an Africa-wide effort to reduce morbidity and mortality associated with the mosquito-transmitted disease.
Malaria is responsible for about 70% of deaths among children in Cameroon, which has a life expectancy at birth of about 57 years.
"The selected vaccine, Mosquirix™, has been chosen by the country based on its pre-qualification, ensuring guaranteed quality, efficacy, and safety for its inclusion in the vaccination programs," as reported by CamarronOnline.org on January 10, 2024.
Malaria vaccines have been approved for use in Africa and are reported to be effective at preventing disease.
Since 2021, the World Health Organization and the European Medicines Agency have recommended the Mosquirix (RTS,S/AS01) malaria vaccine.
In late 2023, the R21 / Matrix-M™ vaccine was WHO-approved for use in certain countries.
As of January 10, 2024, the U.S. FDA had not approved a malaria vaccine.

Atea Pharmaceuticals, Inc. today announced an achievement from its Hepatitis C Virus (HCV) programs. The Company recently reported positive initial data from the first 52 patients in the lead-in cohort of its Phase 2 combination 8-week study of bemnifosbuvir and ruzasvir (RZR) for the treatment of HCV.
"We are excited to share that the initial data from the Phase 2 combination 8-week study showed a 98% Sustained Virologic Response at Week #4, which exceeds our efficacy criterion of >90% for continuing the study. Based on these data, we plan to imminently reinitiate enrollment to complete the Phase 2 study, and topline results are anticipated in the third quarter of 2024," said Jean-Pierre Sommadossi, Ph.D., Chief Executive Officer and Founder of Atea, on January 8, 2024.
"While direct-acting antivirals have transformed HCV treatment, substantial unmet needs still exist, and the rate of new and reinfection currently exceeds cure rates in the U.S. where 2.4 million individuals are estimated to be infected."
"The key unmet needs identified by healthcare providers in market research recently conducted by Atea include shorter length of treatment with fewer contraindications, particularly drug-drug interactions."
The full, unedited press release is available at this company link.
Hepatitis C is a liver infection caused by HCV that is spread through contact with blood from an infected person.
According to the U.S. CDC, for some people, hepatitis C is a short-term illness, but for more than half of people who become infected with the hepatitis C virus, it becomes a long-term, chronic infection.
Chronic hepatitis C can result in serious, even life-threatening, health problems like cirrhosis and liver cancer.
Most health agencies encourage people at risk to get tested.
The CDC's website confirmed on January 10, 2024, that there is no vaccine for hepatitis C.

In late 2023, the World Health Organization issued its first-ever prequalification approval for a vaccine under its Emergency Use Listing (EUL) regulatory pathway, the novel oral polio vaccine type 2 (nOPV2).
Since this next-generation vaccine rollout began in March 2021, the Global Polio Eradication Initiative (GPEI) reported about 1 billion nOPV2 doses have been administered across 35 countries, protecting children against disease.
WHO prequalification will enable additional countries to access the vaccine in response to outbreaks of type 2 variant poliovirus (cVDPV2).
As of January 3, 2024, the GPEI reported that 325 cases of cVDPV2 had been confirmed in 2023, compared to 689 cases in 2022.
While nOPV2 has played a vital part in this reduction, its success, like any polio vaccine, depends on the ability to implement high-quality immunization campaigns that reach every child rapidly, says the GPEI.
"This is a historic milestone for polio eradication and public health," commented WHO Director-General Dr Tedros Adhanom Ghebreyesus in a press release.
"Novel oral polio vaccine type 2 has blazed a trail for other new vaccines that address critical health emergencies, and its use demonstrates the utility of the EUL mechanism in helping to rapidly get new products to where they're needed most."
The nOPV2 vaccine is genetically more stable than existing oral polio vaccines, with a lower risk of reversion to neurovirulence. In addition, this nOPV2 vaccine produces a gut reaction that stops virus transmission using a more stable version of the OPV, which is much less likely to cause paralysis.
The WHO's EUL is reserved for using yet-to-be-licensed vaccines, medicines, and diagnostic tools during public health emergencies like polio outbreaks.
As of January 10, 2024, the nOPV2 vaccine is unavailable in the United States.
According to a recent U.S. CDC Travel Health Notice, over 30 countries reported polio outbreaks in 2023.

The Philadelphia Health Department reported on January 9, 2024, two additional confirmed measles cases, increasing the total number of confirmed measles cases to eight.
In response, the City is working to identify everyone who may have been exposed, checking their vaccine status, warning them that they may have been exposed, and issuing quarantine and exclusion recommendations where necessary.
Philadelphia has expanded the number of potential measles virus exposure locations and dates to account for what has been learned during the ongoing case investigation.
The Health Department’s measles blog post contains the complete list of locations.
The Health Department continues to offer the measles, mumps, and rubella (MMR) vaccines for free at City Health Centers. And the City is offering walk-in MMR vaccinations at three City health centers for a limited time.
Philadelphia and Jefferson Health initially notified the public of this health risk on December 23, 2023.

HilleVax, Inc. and Chengdu Kanghua Biological Products Co., Ltd. today announced the entry into an exclusive license agreement for rights to Kangh's hexavalent virus-like particle (VLP) vaccine candidate for norovirus.
Referred by HilleVax as HIL-216, outside of Greater China, this VLP targets six of the most common norovirus genotypes, including GI.1, GII.2, GII.3, GII.4, GII.6, and GII.17.
HilleVax stated it intends to launch a Phase 1 trial in 2024.
According to the press release on January 8, 2024, the Investigational New Drug application for HIL-216 was cleared by the U.S. FDA in September 2023.
As of January 9, 2024, the FDA has not approved any norovirus vaccine candidate for use in the U.S.
Rob Hershberg, MD, Ph.D., Chairman and Chief Executive Officer at Hillevax, commented, "Our bivalent norovirus VLP vaccine candidate, HIL-214, remains the most advanced norovirus vaccine candidate in clinical development, and we are on track to report topline safety and efficacy data in mid-2024."
"We believe that HIL-214 will be the first norovirus vaccine submitted for registration and, if approved, would address the significant unmet medical need in infants and other at-risk populations."
"We further believe that HIL-216 is an exciting addition to the HilleVax portfolio as a next-generation, higher valency VLP-based vaccine and is an ideal fit with the expertise, capabilities, and long-term aspirations of HilleVax."
HilleVax confirmed it will pay Kangh an upfront payment of $15 million with the potential for additional payments of up to $255.5 million upon achieving specific development and sales milestones. Kangh can also receive a single-digit tiered royalty on net sales outside of Greater China.
Globally, norovirus is estimated to result in approximately 700 million cases of acute gastroenteritis and 200,000 deaths per year, resulting in over $4 billion in direct health system costs and $60 billion in societal costs per year.
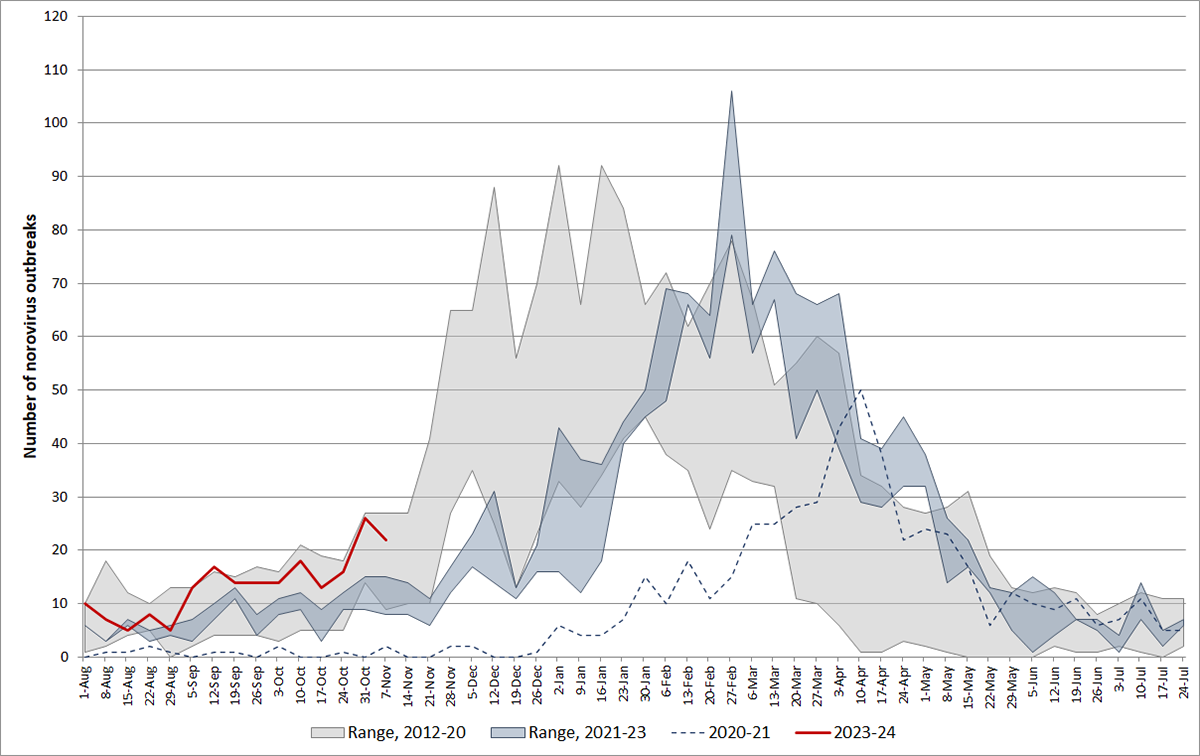
According to the U.S. CDC, norovirus is a contagious virus that causes vomiting and diarrhea. Anyone can get infected and sick with norovirus. Most norovirus outbreaks in the U.S. happen from November to April.
From August through November 13, 2023, there were 202 norovirus outbreaks reported by NoroSTAT-participating states. During the same period last season, 134 norovirus outbreaks were reported by these states, according to the CDC.
HilleVax is a clinical-stage biopharmaceutical company based in Boston, MA, focused on developing and commercializing novel vaccines.