Search API
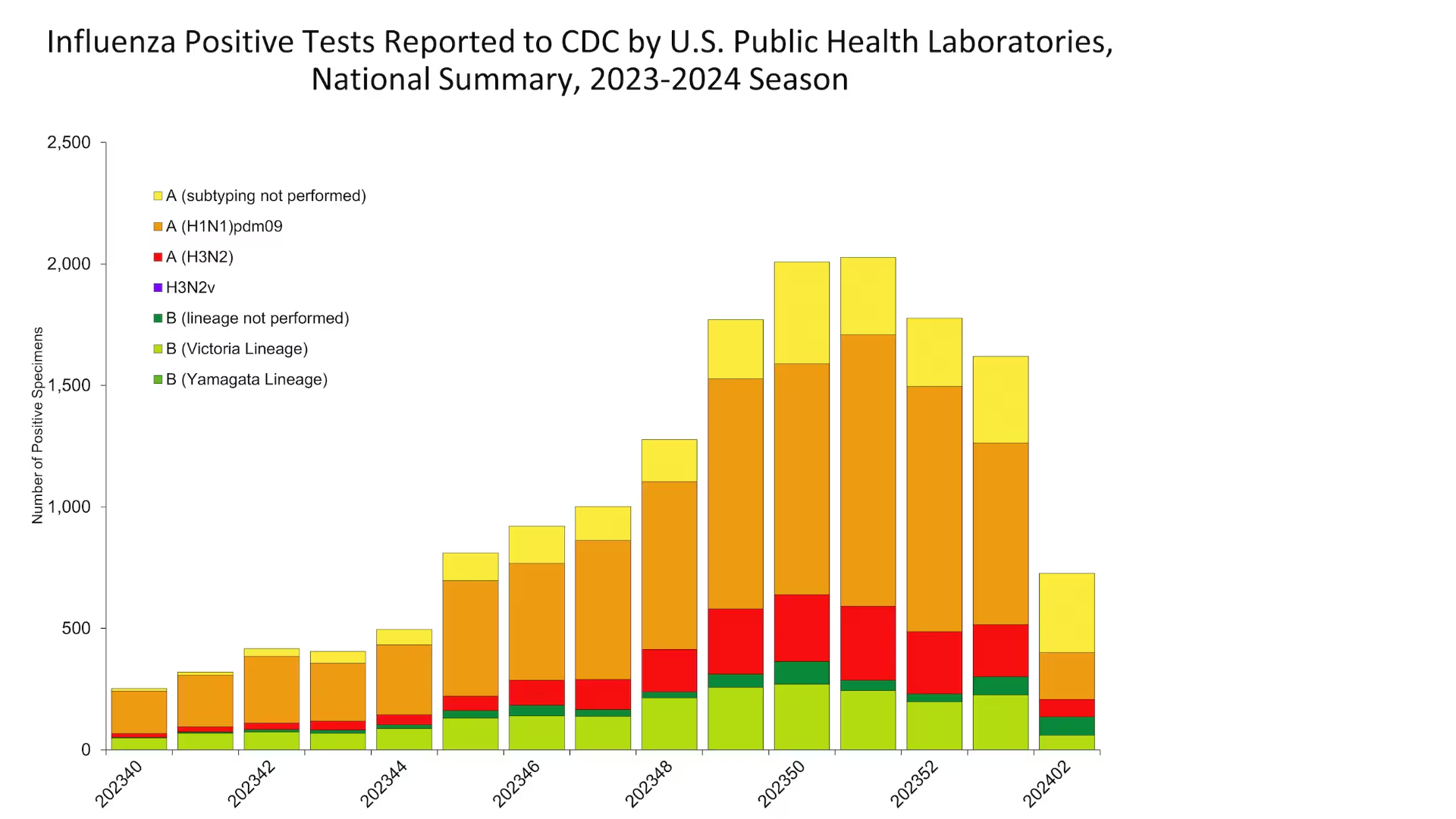
The Global Polio Eradication Initiative (GPEI) recently reported 20 circulating vaccine-derived poliovirus type 1 or type 2 (cVDPV2) cases were reported in six African countries in late 2023.
Chad, the Democratic Republic of the Congo, Ivory Coast, Mozambique, Nigeria, and South Sudan reported new polio cases.
Since March 2021, the GPEI has helped administer nearly 1 billion doses of novel oral polio vaccine type 2 (nOPV2) across 35 countries, primarily in Africa.
After nearly three years of use, estimates show that nOPV2 is 80% less likely to seed new variant polio outbreaks, making it the tool of choice to stop these outbreaks for good, says the GPEI.
The World Health Organization (WHO) confirmed in December 2023 that the spread of the poliovirus (31 countries) remained a Public Health Emergency of International Concern.
The WHO Director-General, Dr Tedros Adhanom Ghebreyesus, commented on January 9, 2024, "nOPV2 has blazed a trail for other new vaccines that address critical health emergencies."
In late December 2023, the WHO issued its first-ever prequalification approval for a vaccine, nOPV2, being used under its Emergency Use Listing regulatory pathway.
As of early January 2024, 325 cases of cVDPV2 had been reported in 2023, compared to 689 cases in 2022.
In the United States, following the detection of poliovirus in New York, the U.S. CDC selected jurisdictions to strategically expand wastewater testing for poliovirus in counties with potentially low polio vaccination coverage.
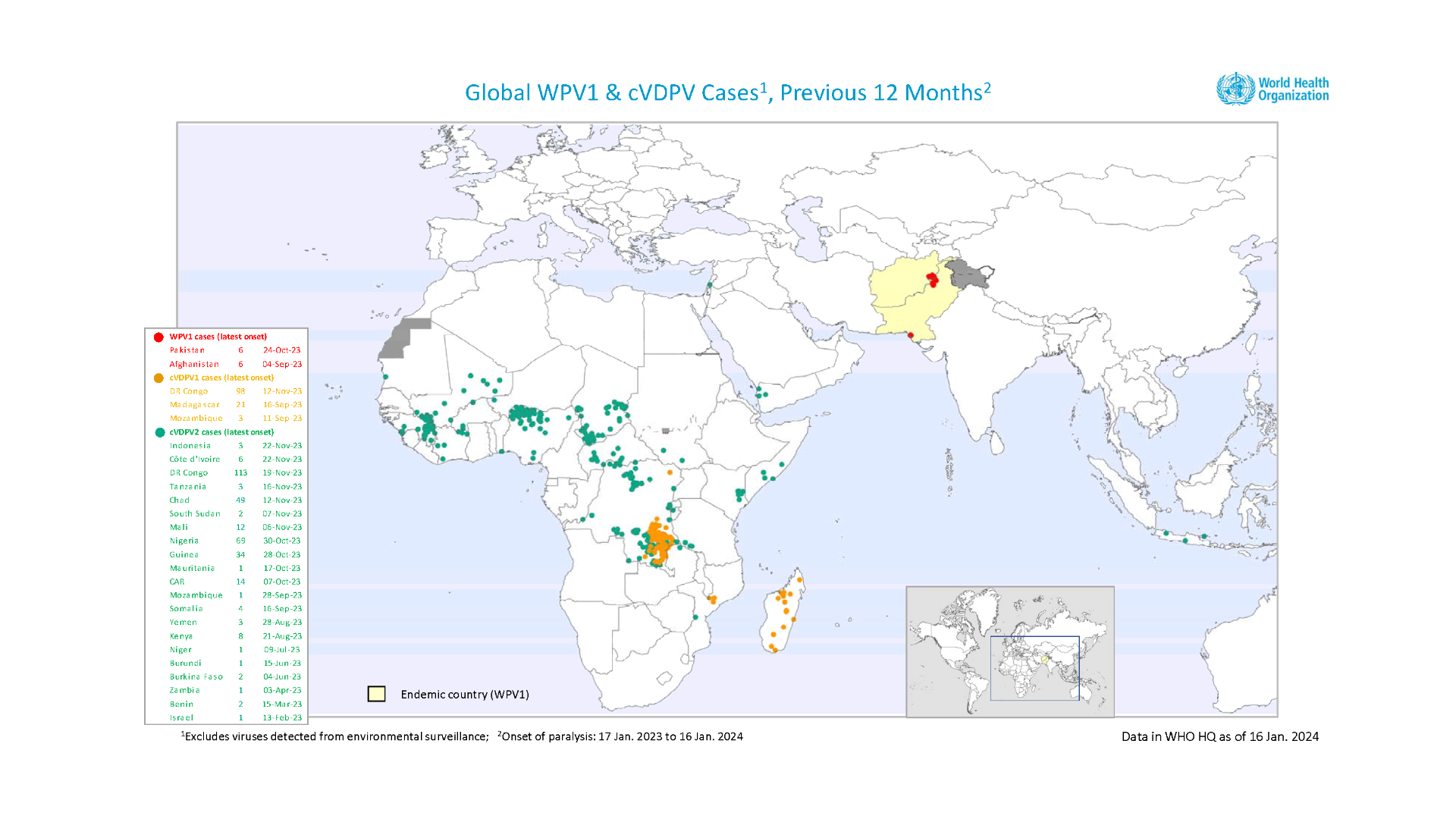
Last year, the World Health Organization (WHO) confirmed the first known case of Western Equine Encephalitis (WEE) in more than two decades in Argentina.
Since then, and until January 9, 2024, 21 human cases in Argentina have been confirmed by the WHO/PAHO.
To alert international travelers to this WEE health risk, the U.S. CDC issued a Level 1 - Practice Usual Precautions, Travel Health Notice on January 12, 2024.
WEE is a rare, mosquito-borne viral disease that affects equines and humans. Most human cases are associated with epidemics in birds or horses.
The CDC says travelers to these regions of Argentina should avoid mosquito bites, which can transmit the Eastern Equine Encephalitis Virus (EEEV).
EEEV transmission is most common in and around freshwater hardwood swamps in the Atlantic and Gulf Coast states and the Great Lakes region in the U.S.
Only about 4-5% of human EEEV infections result in EEE.
In the United States, an average of 11 human cases of EEE are reported annually, according to the CDC. There were 7 EEEV neuroinvasive disease cases in 2023, reported by four states.
From 2012-2021, most cases of EEE have been reported from Massachusetts, Michigan, Florida, Georgia, and North Carolina.
As of 2024, the CDC says no vaccine prevents EEE virus infection.
However, the U.S. Army Medical Research Institute of Infectious Diseases developed a human vaccine for EEE in the mid-1980s, but it has never been approved for public use.
The U.S. government recently invested in a vaccine against western, eastern, and Venezuelan equine encephalitis viruses.
MVA-BN® WEV has completed Phase 1 clinical development demonstrating potential for broad, and long-term protection, with a Phase 2 study planned for 2024.
According to various news sources, Indian Immunologicals Ltd (IIL) launched Havisure, the 'first' indigenously developed two-dose Hepatitis A vaccine.
"Currently, Hepatitis A vaccines are imported into our country, and as a true meaning of Atma Nirbhar Bharat, IIL has tirelessly put in efforts and developed India's 1st vaccine for Hepatitis A," said K Anand Kumar, Managing Director, IIL, told newspersons such as Businessline on January 19, 2024.
Priced at ₹2,150 ($25) per dose, the vaccine is recommended for individuals at risk of exposure or travel to regions with high hepatitis A prevalence. In addition to this, people with occupational risk of infection and suffering from chronic liver diseases also need Hepatitis A vaccination.
According to the U.S. CDC, Hepatitis A is a severe liver disease. It is usually spread through close, personal contact with an infected person or when a person unknowingly ingests the virus from objects, food, or drinks contaminated by small amounts of stool from an infected person.
Hepatitis A vaccinations (Havrix, Twinrix, VAQTA) have made this disease much less common in the United States.
However, outbreaks of hepatitis A among unvaccinated people still happen. Since hepatitis A outbreaks were first identified in 2016, the World Health Organization estimates that 7,134 persons have died from hepatitis A worldwide.
In the U.S., 37 states have publicly reported the following cases as of January 12, 2024:
Cases: 44,947
Hospitalizations: 27,469 (61%)
Deaths: 424
In response to all hepatitis outbreaks, the CDC provides ongoing epidemiology and laboratory support and support on vaccine supply and vaccine policy development.

The Costa Rica Ministry of Health today announced it confirmed a measles case near the capital city of San Jose.
This new measles patient was detected on January 11, 2024. In 2023, one measles patient was also confirmed.
Given this new measles case, the Costa Rica health authorities conducted all follow-up efforts to address the situation. Currently, this person is in good health.
An active community search for cases in San Josecito de San Rafael de Heredia was conducted on January 18, 2024. As a result of the search, no additional measles cases were found, as most of the people approached had completed vaccination schedules. A total of 408 houses were visited.
After the WHO Region of the Americas was declared measles-free in September 2016, a steady increase in imported measles cases from other WHO Regions and between countries within the Region of the Americas was observed.
For example, several states in the eastern United States have reported measles cases in early 2024.
Last year, the Pan American Health Organization / World Health Organization (PAHO/WHO) urged Member States to continue activities to increase and maintain adequate vaccination coverage against measles, rubella, and mumps (MMR).
Globally, the WHO has reported significant measles outbreaks throughout 2023.
In addition to measles, Costa Rica confronted an expansion of Dengue and Zika cases in 2023.
Vaxart, Inc. today announced that the United States Biomedical Advanced Research and Development Authority (BARDA) has awarded the Company $9.27 million to fund preparation for a 10,000-subject Phase 2b clinical study evaluating Vaxart's oral pill XBB COVID-19 vaccine candidate against an approved mRNA vaccine comparator.
Vaxart's oral pill vaccine platform provides many of the features desired by BARDA, such as generating mucosal immunity and providing a cross-reactive response to many COVID variants.
"We believe we have the chance to improve on existing vaccines in two important ways," said Dr. James F. Cummings, Vaxart's Chief Medical Officer, in a press release on January 19, 2024.
"First, a thermostable pill vaccine such as Vaxart's offers the chance to overcome needle-phobia, a documented obstacle to vaccination, and offers the potential to make it easier to vaccinate more people faster than traditional injected vaccines."
"Second, our previous research on other vaccine constructs found Vaxart's oral pill vaccine to be cross-reactive against all tested SARS-CoV-2 variants and to trigger long-lasting immune responses, potentially offering broader, longer protection than the current first-generation vaccines."
"We believe our vaccine does this by triggering both a systemic and mucosal response."
This oral vaccine development project has been funded with federal funds from the U.S. Department of Health and Human Services (HHS). Project NextGen is a $5 billion initiative by HHS to develop new, innovative vaccines and therapeutics that provide broader and more durable protection against COVID-19 than the first-generation COVID vaccines and medicines.
Project NextGen is separate but similar to the Disease X initiative.

As the 2024 winter season begins to blanket the United States in snow, the Centers for Disease Control and Prevention today published updated insights regarding influenza vaccinations.
As of January 19, 2024, the CDC reported the percentage of the overall population who received one of the nine approved influenza vaccines was 47.5% (95% confidence interval).
From an age segmentation, the CDC reported the following:
- 46.1-48.8 for children,
- 46.7% (45.8-47.6) for adults 18+,
- 73.0% (70.8-75.2) among adults age 65+.
From an availability perspective, the CDC previously reported that over 155 million flu vaccines (egg, cell, and nasal-based) had been distributed during the 2023-2024 season, which are generally available at most pharmacies in the U.S.
Additionally, the CDC's FluView Updates for Week #2, ending January 13, 2024, stated that after several weeks of increases in key flu indicators through the end of 2023, two weeks of decreasing or stable trends nationally have been noted.
However, seasonal influenza activity remains elevated in most parts of the country.
Furthermore, the CDC is monitoring for a second period of increased influenza activity that often occurs after the winter holidays.
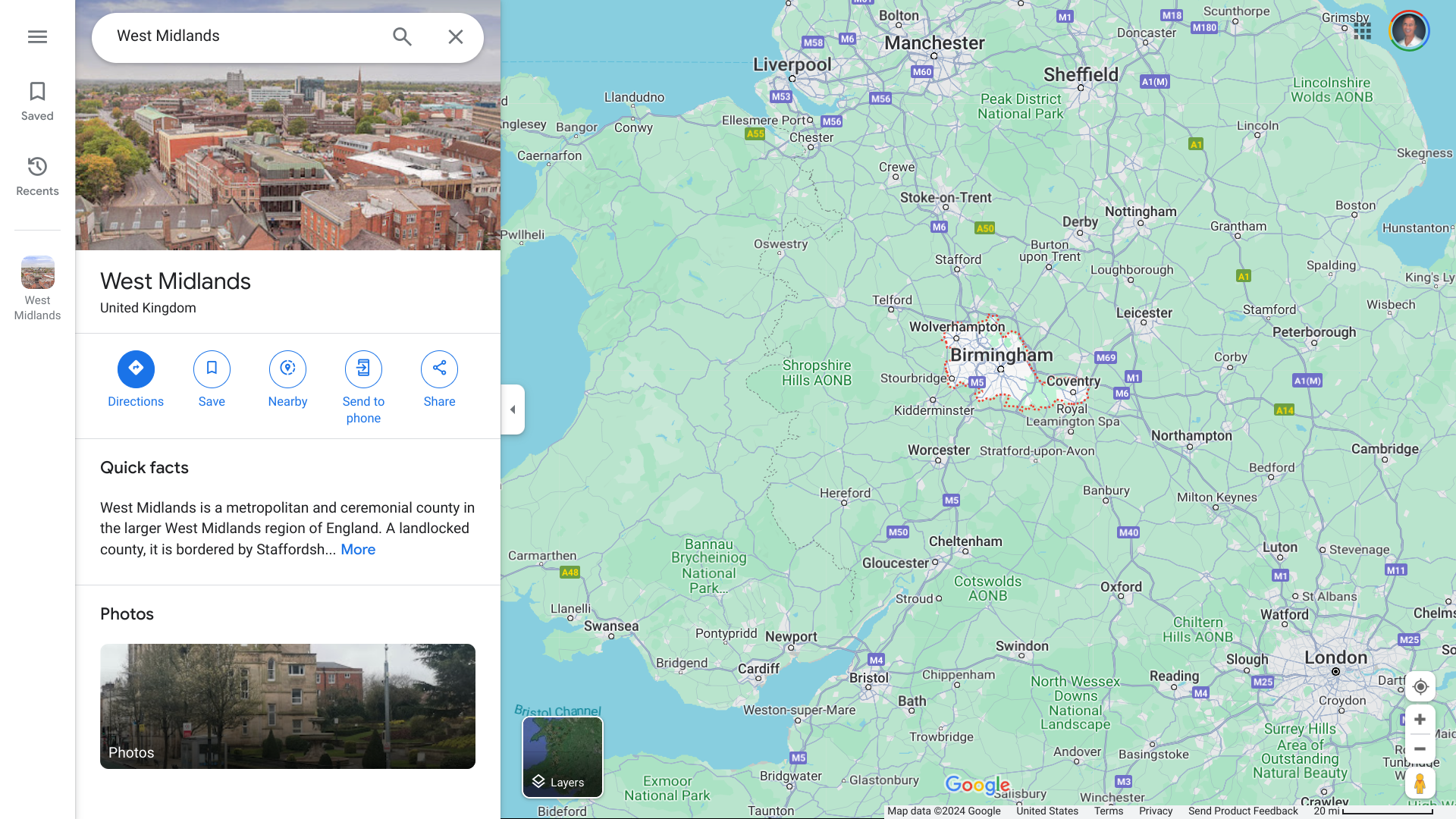
When the U.S. Centers for Disease Control and Prevention issued a Level 1 - Practice Usual Precautions, Travel Health Notice, regarding measles outbreaks in 47 countries during November 2023, it did not include the United Kingdom.
Unfortunately, the U.K. Health Security Agency (UKHSA) has declared a national measles incident.
The UKHSA's Chief Executive has confirmed a recent increase in measles cases in parts of England's West Midlands region.
As of January 18, 2024, 216 confirmed measles cases and 103 probable cases have been reported in the West Midlands since October 2023.
Around 80% of cases have been seen in Birmingham, with about 10% in Coventry.
This area is located northwest of London.
There were 1,603 suspected cases of measles in England and Wales in 2023, according to the UKHSA.
Professor Dame Jenny Harries, Chief Executive of UKHSA, said in a press release, "Colleagues across the West Midlands have worked tirelessly to try to control the (measles) outbreak, but with vaccine uptake in some communities so low, there is now a very real risk of seeing the virus spread in other towns and cities."
"The MMR vaccine is the best way for parents to protect their children from measles."
"Immediate action is needed to boost MMR uptake across communities where vaccine uptake is low. We need a long-term concerted effort to protect individuals and to prevent large measles outbreaks."
In December 2023, NHS England announced a new Vaccination Strategy, which includes tactics to combat measles outbreaks.
In the U.S., several cities/states have confirmed measles cases related to unvaccinated people in early 2024. Most measles cases in the U.S. are related to international travelers.
Measles spreads very quickly among those who are unvaccinated, especially in nurseries and schools. The MMR vaccine is a safe and effective way of protecting against measles, mumps, and rubella.
MMR vaccines are generally available in clinics and pharmacies in the U.S.
Combating Antibiotic-Resistant Bacteria Biopharmaceutical Accelerator (CARB-X) today announced it is awarding $1.7 million to Syntiron to develop a maternal vaccine that targets Escherichia coli and Klebsiella pneumoniae.
Syntiron's Alloy-EK vaccine leverages iron receptor proteins (IRPs) as vaccine targets. IRPs are highly genetically conserved, which means they are less likely to change since they perform essential functions.
This makes these proteins reliable vaccine targets.
Syntiron developed the Alloy Platform to manufacture and formulate IRP vaccines safely that maintain robust immunity and cover a broad range of bacterial strains.
Neonatal sepsis is a life-threatening response to bloodstream infections that occur in newborns fewer than 28 days old. Due to their immature immune systems, newborns are particularly susceptible to infections.
Since neonatal sepsis progresses rapidly, the risk of death from neonatal sepsis increases by 7.6% every hour a treatment is delayed.
The BARNARDS study estimated that 2.5 million neonates or infants die in the first month of life of sepsis annually, with the most significant burden in low- and middle-income countries.
"The bacteria targeted by this vaccine are a tremendous burden on public health for babies, children, and adults worldwide," said Lisa Herron-Olson, Ph.D., Managing Director of Syntiron, in a press release on January 18, 2024.
"Pregnant women are at particularly high risk of infection by these same bacteria that can cause neonatal sepsis in newborn babies."
"Preventing these infections by vaccination offers substantial potential health benefits to mothers and babies while reducing the spread of antimicrobial resistance."
Syntiron's innovation with the Alloy Platform leveraged molecular, bioinformatic, and immunological principles to engineer simpler proteins enriched in linear, immunodominant, and highly conserved peptides derived from IRPs.
Founded in 2004, Syntiron is a biotechnology company based in Saint Paul, MN, and has several vaccines in development that target bacterial infections.=

A non-peer-reviewed study supported by the U.S. Food and Drug Administration (FDA) as part of the SafeRx Project, a joint initiative of the Centers for Medicare & Medicaid Services, published on January 17, 2024, did not observe clear, consistent evidence of increased stroke risk following high to dose or adjuvanted influenza vaccination across the three flu seasons 2016 to 2019.
The statistically significant associations identified in this study were not consistently observed across outcomes, risk windows, age subgroups, or seasons.
Furthermore, the clinical significance of any potential risk of stroke following influenza vaccination must be carefully considered, together with the significant benefits of receiving an annual flu shot.
While studies have shown that influenza infections increase the risk of stroke, this study only included vaccinated cases and did not account for the protective effect of vaccination against infections. Address correspondence to Yun Lu, MS, PhD, Silver Spring, MD 20993; email: [email protected].