Search API
The Coalition for Epidemic Preparedness Innovations (CEPI) today announced Serum Institute of India Pvt. Ltd (SII) has joined CEPI's network of vaccine producers in the Global South to support more rapid, agile, and equitable responses to future public health disease outbreaks.
To prepare for such a scenario, CEPI is investing up to $30 million to build upon SII's proven track record of rapid response to infectious disease outbreaks, expanding the company's ability to swiftly supply investigational vaccines in the face of epidemic and pandemic threats.
Created by CEPI to expand the global footprint of vaccine production, the manufacturing network focuses on vaccine makers in the Global South near areas at high risk of outbreaks caused by deadly viral threats like Lassa Fever, Nipah, Disease X, and other pathogens with epidemic or pandemic potential prioritized by CEPI.
Dr. Richard Hatchett, CEO of CEPI, said in a press release on January 23, 2024, "As part of CEPI's global manufacturing network, SII's world-renowned manufacturing and rapid response capabilities are poised to play a critical role in enabling swift and equitable access to affordable outbreak vaccines for the Global South."
Given SII's already proven production capabilities, the company may be called upon to promptly supply investigational vaccines for preclinical and clinical testing and large-scale supply in the event of an outbreak.
Shortening the time taken to manufacture and validate the first batches of experimental vaccines will be vital to enabling a response to an escalating outbreak within just 100 days – a goal created by CEPI and embraced by the G7, G20, and industry leaders – and could help stop a future pandemic in its tracks.

The WHO European Region confirmed today the measles virus spread in 41 of its 53 Member States in 2023. Among the countries most affected in the Region, the Republic of Kazakhstan has recorded the highest incidence, with 13,677 measles cases in 2023.
The majority of Kazakhstan measles cases were in children, 11,300.
As of January 23, 2024, there are 2,167 children in a Kazakhstan hospital with measles, 27 of them in a serious condition.
To implement measles vaccination campaigns, the Kazakhstan government purchased an additional 1.5 million doses of MMR vaccine in 2023.
"We have seen in the Region not only a 30-fold increase in measles cases ....This is concerning," explained Dr. Hans Henri P. Kluge, WHO Regional Director for Europe, in a press release in 2023.
"Vaccination is the only way to protect children from this potentially dangerous disease."
"Urgent vaccination efforts are needed to halt transmission and prevent further spread."
"It is vital that all countries are prepared to rapidly detect and respond promptly to measles outbreaks, which could endanger progress towards measles elimination," added Dr. Kluge.
In the United States, several cities, such as Atlanta, Kansas City, Philadelphia, and Wilmington, have reported measles cases in 2024.
The U.S. CDC says most measles cases in the U.S. are travel-related. In 2023, there were 56 measles cases reported by 20 U.S. jurisdictions.
Measles is a vaccine-preventable disease. Various measles vaccines are available at local pharmacies in the U.S.

HDT Bio Corp. today announced that it has partnered with the Biomedical Advanced Research and Development Authority (BARDA), under Project NextGen's Enabler's program.
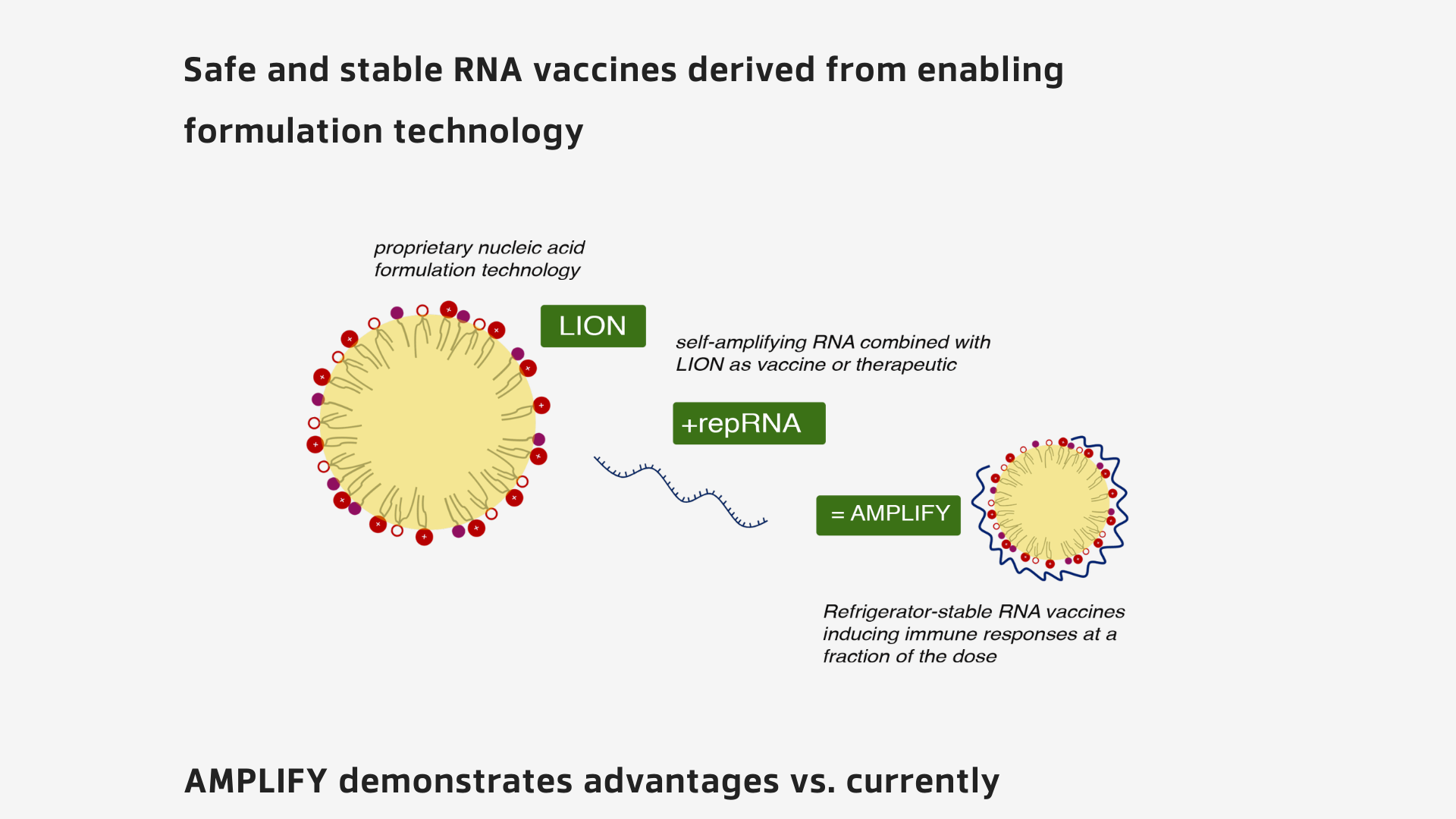
Through this BARDA partnership, HDT Bio has received a $749,000 contract, supporting proof-of-concept studies for on-demand manufacturing and release processes that use HDT Bio's LION™ formulation for RNA vaccine production.
These studies will provide proof-of-concept data on the LION platform in support of on-demand manufacturing and release processes that enable rapid production of 1000 repRNA vaccine doses in seven days.
LION is a proprietary nanoemulsion tailored for RNA-based vaccines and therapeutics production designed to enable facile formulation and faster manufacturing compared to the characteristics of standard lipid nanoparticles used in existing vaccine products.
"We look forward to contributing to the broader access to treatment and prevention strategies of future infectious diseases," commented Steven Reed, Ph.D., Co-founder and Chief Executive Officer of HDT Bio, in a press release on January 23, 204.
With an initial investment of $5 billion, Project NextGen intends to accelerate and streamline the development of the next generation of vaccines and treatments through public-private collaborations.
The Project NextGen Enabler's program aims to advance next-generation vaccine and therapeutics technologies, including the development of innovative cGMP manufacturing of vaccines that decrease costs, speed production, increase efficacy, and improve access.
Previous BARDA - Project NextGen awards include, but are not limited to, $8.5 million to CastleVax for a vector-based intranasal vaccine candidate, $10 million to Gritstone Bio for a self-amplifying mRNA vaccine candidate, and $9.27 million to Vaxart's evaluation of its oral pill XBB COVID-19 vaccine candidate.
Project NextGen is separated from the Disease X initiative.
The Republic of Cameroon today launched the Mosquirix™ (RTS,S) malaria vaccine into its routine national immunization services, becoming the first country to do so outside the malaria vaccine pilot program previously carried out in the African countries of Ghana, Kenya, and Malawi.
The launch on January 22, 2024, came after Cameroon received 331,200 Mosquirix doses in November 2023. Additional malaria vaccines are expected in 2024.
“The vaccine is an additional tool for malaria control. The country has chosen it (Mosquirix) based on its pre-qualification, ensuring guaranteed quality, efficacy, and safety for inclusion in the vaccination program,” said Dr. Shalom Ndoula, Permanent Secretary of the Expanded Programme on Immunization in Cameroon, in a press release.
“It will specifically target all children aged six months as of December 2023.”
In 2023, the Pan American Health Organization estimated that approximately 41 million people in twenty-one Latin American countries are at risk for malaria.
In the U.S., the Centers for Disease Control and Prevention confirmed about ten locally-acquired malaria cases in 2023.
Two approved malaria vaccines are available in certain countries in 2024 but not in the United States.
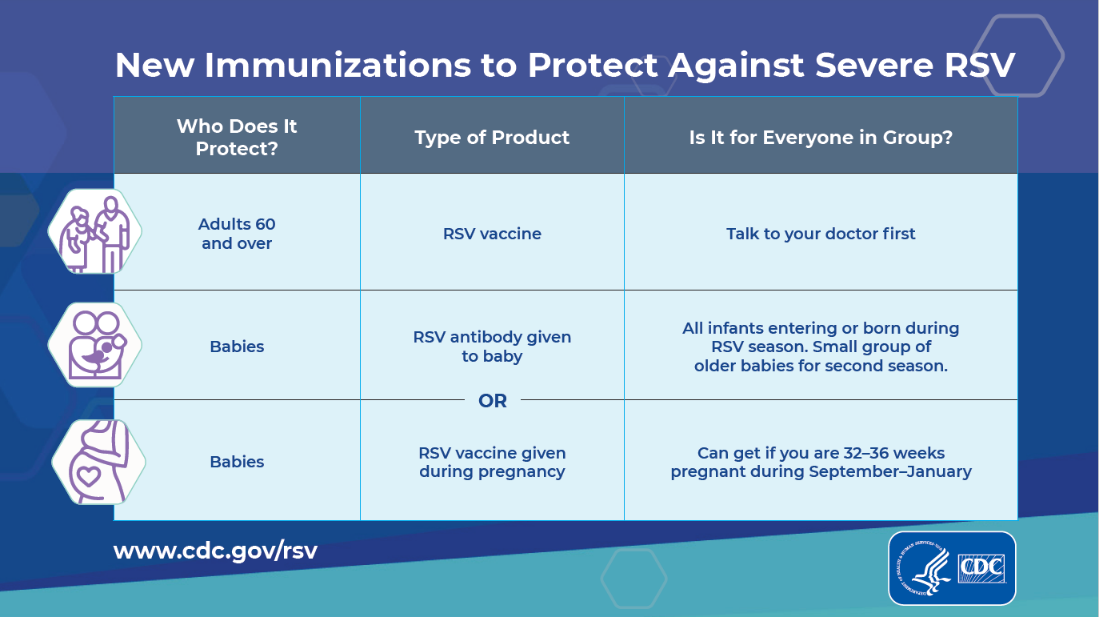
Since the approval of Respiratory Syncytial Virus (RSV) vaccines and an enhanced monoclonal antibody in 2023, the U.S. Centers for Disease Control and Prevention (CDC) and the Food and Drug Administration (FDA) have received reports of RSV vaccines being administered in error to young children and pregnant women.
A Clinician Outreach and Communication Activity COCA Now email issued today says the number of reports received by the Vaccine Adverse Event Reporting System (VAERS) as of January 17, 2024, suggests that these types of errors are uncommon in young children less than two years of age (25 reports) and pregnant women (128 reports).
The CDC stated this is a relatively small amount of errors compared to the estimated one million infants protected from RSV either through infant receipt of Beyfortus™ (nirsevimab) or by pregnant women being vaccinated.
According to the CDC, most of these administration error reports described no adverse event. When an adverse event was concurrently reported to VAERS, most reports were classified as nonserious.
The CDC, FDA, and other federal agencies said they continue to monitor the safety of RSV vaccines and reports of vaccine administration errors and will share information with the public as it becomes available.
On January 22, 2024, the CDC published Recommendations for Healthcare Providers who Have Administered Incorrect RSV Vaccine Products to Their Patients.
The U.S. Centers for Disease Control and Prevention (CDC) recent influenza vaccination coverage estimates indicate a continued decrease among pregnant women since 2020.
Based on data from January 13, 2024, the CDC reported last Friday that flu vaccination coverage among pregnant Women as of December 2023 is about 3% lower compared to the end of December 2022 (36% Vs. 39%),
And about 6% lower compared to the end of December 2021 (36% Vs. 42%) and 17% points lower than at the end of December 2020 (36% Vs. 53%).
With the 2023-2024 flu season ongoing in the United States and numerous countries, the CDC recommends flu vaccination and early antiviral treatment for certain people.
There’s still time to benefit from the protection vaccination offers this flu season, says the CDC.
Various flu shots remain available at health clinics and pharmacies in the U.S.

As the world awaits the approval of a Human Immunodeficiency Virus (HIV) vaccine, a novel candidate based on Gorilla adenoviral vector (GRAd-HIV) technology was recently funded by The Bill & Melinda Gates Foundation.
Over the past few decades, various HIV vaccine candidates have not succeeded in human clinical trials.
Announced on January 22, 2024, ReiThera Srl, the Ragon Institute of Mass General, MIT, Harvard, and IAVI confirmed a collaboration to develop a novel HIV vaccine candidate that will be composed of ReiThera’s GRAd vector and HIV T-cell epitopes.
ReiThera’s vaccine platform uses a novel proprietary GRAd vector belonging to species C adenoviruses that are considered among the most potent vaccine carriers for the induction of CD8 T-cell responses to the encoded antigens and having a low seroprevalence in humans.
Prior findings by the Ragon Institute have shown that mutation of residues at important network positions disproportionately impaired viral replication and occurred with high frequency in epitopes presented by protective human leukocyte antigen (HLA) class I alleles.
Moreover, CD8+ T-cell targeting of highly networked epitopes distinguished individuals who naturally control HIV, even in the absence of protective HLA alleles.
“We are thrilled to have the opportunity to collaborate with ReiThera and IAVI, with the support of the Bill & Melinda Gates Foundation, to advance the GRAd-HIV highly networked T-cell vaccine candidate towards clinical evaluation,” said Gaurav Gaiha, Ragon faculty member, in a press release.
“We are particularly pleased that this takes place with partners in sub-Saharan Africa, given the immense need for new solutions to curtail the ongoing HIV epidemic.”
Key partners in this program include researchers at the Africa Health Research Institute, the National Institute for Communicable Diseases in South Africa, Mutala Trust, and Charles River Medical Group in Zimbabwe.
IAVI is the sponsor and will execute a phase I clinical trial.