Search API
According to the U.S. CDC Yellow Book 2024, an international traveler's risk for acquiring Yellow Fever virus is determined by their immunization status and destination-specific and travel-associated factors.
Since about thirty countries require proof of a pre-arrival yellow fever vaccination, many travelers have questions about the vaccine's long-term efficacy.
On January 22, 2024, the Lancet Global Health recently published results from a systematic review aimed at assessing the necessity of a booster vaccination based on the long-term (10+ years) immunogenicity of primary yellow fever vaccination in travelers and in residents of yellow fever-endemic areas, as well as in specific populations, including children and immunocompromised individuals.
The gathered evidence suggested that a single dose of yellow fever vaccine provides lifelong protection (overall seroprotection rate 94%) in travelers.
However, in people living with HIV and young children (<2 years), booster doses might still be required because lower proportions of vaccinees were seroprotected ten or more years post-vaccination.
The pooled seroprotection rate was 47% in children and 61% in people living with HIV.
Lower observed seroprotection rates among residents of yellow fever endemic areas were partly explained by the use of a higher cutoff for seroprotection that was applied in Brazil. No conclusions could be drawn for the sub-Saharan Africa region.
The CDC says most people infected with yellow fever do not get sick or have only mild symptoms. People who get sick will start having symptoms 3–6 days after infection.
According to the CDC, about 12% of people with symptoms develop serious illnesses.
The study was registered with PROSPERO, CRD42023384087. No industry conflicts of interest were disclosed.

The journal Clinical Microbiology and Infection published the results from a post-hoc analysis regarding the relative effectiveness of high-dose quadrivalent influenza vaccine (QIV-HD) vs. standard-dose quadrivalent influenza vaccine (QIV-SD) against recurrent hospitalizations for seniors.
Published on January 27, 2024, this analysis found that among 12,477 randomly assigned participants, receiving QIV-HD was associated with lower incidence rates of hospitalisations for pneumonia or influenza (10 vs. 33 events, IRR 0.30 [95% CI 0.14-0.64], p=0.002).
And all-cause hospitalisations (647 vs. 742 events, IRR 0.87 [95% CI 0.76-0.99], p=0.032) compared with QIV-SD.
These researchers wrote, 'Our exploratory results correspond to a number needed to treat 65 (95% CI 35-840) persons vaccinated with QIV-HD compared with QIV-SD to prevent one additional all-cause hospitalization per season.'
'Further research is needed to confirm these hypothesis-generating findings.'
As of January 29, 2024, over 156 million cell, egg, and nasal-based influenza vaccines were distributed in the U.S. during the 2023-2024 flu season.

Anyone can get pneumococcal disease, but some people are at increased risk. To better protect children and seniors from disease, innovative vaccine candidates are conducting clinical trials in 2024.
Currently, two kinds of pneumococcal vaccines are recommended in the U.S. - Pneumococcal conjugate vaccines (PCVs, specifically PCV15 and PCV20) and Pneumococcal polysaccharide vaccine (PPSV23).
However, even with U.S. FDA-approved vaccines broadly available, approximately 5,000 deaths are related to pneumococcal disease each year in the U.S.
To address this health issue, Vaxcyte, Inc. today announced the completion of enrollment in its Phase 1/2 clinical study evaluating VAX-31, a next-generation 31-valent PCV and the broadest-spectrum pneumococcal vaccine candidate in the clinic today.
This vaccine candidate is designed to prevent invasive pneumococcal disease (IPD).
Vaxcyte expects to announce topline safety, tolerability, and immunogenicity data from the Phase 1/2 study in the third quarter of 2024.
"Completing the enrollment of the VAX-31 study with more than one thousand adults 50 years and older is a significant step for our PCV franchise, and we look forward to announcing topline safety, tolerability, and immunogenicity data in the third quarter of this year," said Grant Pickering, Chief Executive Officer and Co-founder of Vaxcyte, in a press release.
"VAX-31, the broadest-spectrum PCV in the clinic, has the potential to address a significant public health need by covering approximately 95% of IPD circulating in the U.S. adult population while maintaining coverage of previously circulating strains that are currently contained via ongoing vaccination."
Vaxcyte's unedited press release is posted here.
In September 2023, the Taiwan Centers for Disease Control (Taiwan CDC) announced that mRNA vaccines would continue to be available in response to the continued spread of COVID-19.
However, according to Novavax Inc.'s announcement on January 23, 2024, the updated protein-based non-mRNA COVID-19 vaccine (Nuvaxovid™XBB.1.5 dispersion for injection) is now available for use in Taiwan for the prevention of COVID-19 in individuals aged 12 and older.
Doses have been distributed by Taiwan CDC to local vaccination clinics across the country.
The Taiwan CDC stated that adding a protein-based vaccine will diversify the country's vaccine portfolio and provide a non-mRNA option to help protect against COVID-19.
"We are working closely with Taiwan's authorities to ensure doses of our updated protein-based non-mRNA COVID-19 vaccine are made available at vaccination centers across Taiwan as soon as possible," said John C. Jacobs, President and Chief Executive Officer, Novavax, in a press release posted on December 18, 2023.
Throughout the multi-year pandemic, about 90% of Taiwan's population contracted COVID-19, and 19,005 people died.
Taiwan's authorization was based on non-clinical data showing that Novavax's updated COVID-19 vaccine induced functional immune responses for XBB.1.5, XBB.1.16, and XBB.2.3 variants.
Additional non-clinical data demonstrated that Novavax's NVX-CoV2601 vaccine-induced neutralizing antibody responses to subvariants BA.2.86, EG.5.1, FL.1.5.1, and XBB.1.16.6 as well as CD4+ polyfunctional cellular (T-cell) responses against EG.5.1 and XBB.1.16.6.
These data indicate Novavax's vaccine can stimulate both arms of the immune system and induce a broad response against circulating variants, says Novavax.

Malaria affects millions of lives annually, particularly in tropical and subtropical regions, according to a Perspective published by the Malaria Journal.
Despite being largely preventable, malaria outbreaks caused 247 million infections and over 600,000 deaths across 85 countries in 2021,
In the ongoing battle against malaria outbreaks, a promising development has emerged with the endorsement by the World Health Organization of the R21/Matrix-M™ Malaria Vaccine.
Developed through a collaboration between the University of Oxford and Novavax Inc., this vaccine has demonstrated remarkable efficacy, reaching 77% effectiveness in Phase 2 clinical trials.
R21 is designed to be low-dose, cost-effective, and accessible, with approval for use in children under three years old.
Published on January 12, 2024, this paper critically examines the R21/Matrix-M malaria vaccine, its development, potential impact on global malaria eradication efforts, and the challenges and opportunities it presents.
The U.S. Centers for Disease Control and Prevention says about 2,000 malaria cases are diagnosed in the United States annually, mostly in travelers returning to cities such as Miami, Florida.
As of January 27, 2024, malaria vaccines are unavailable in the U.S.

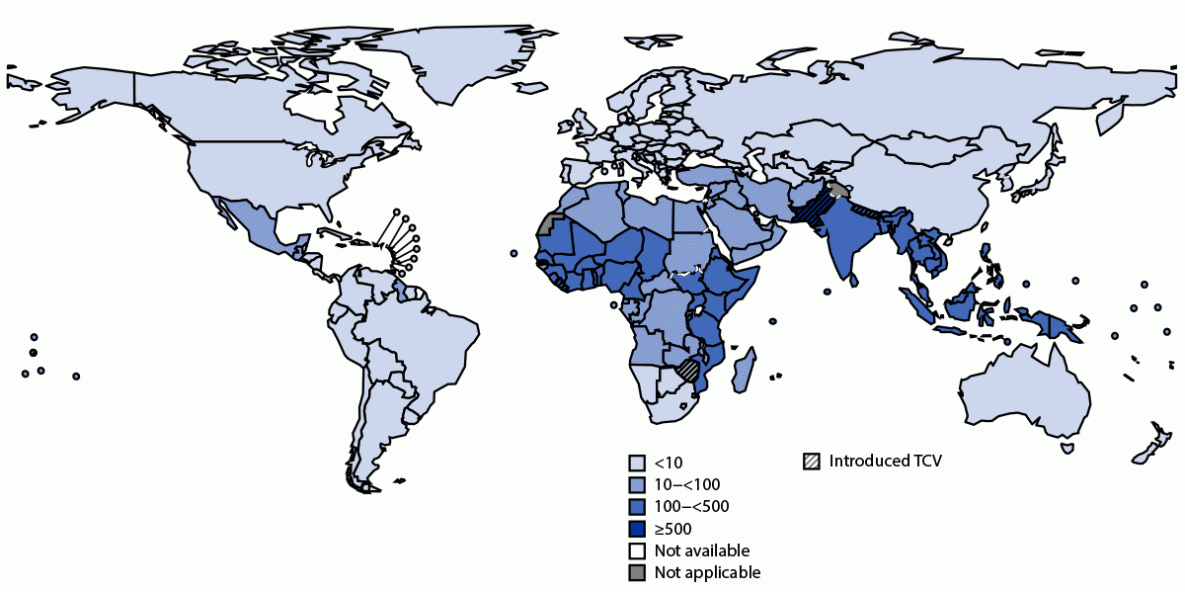
A recent study funded by the Bill & Melinda Gates Foundation found that a single-dose vaccine against typhoid was efficacious for at least four years among children in all age groups.
The Vi polysaccharide conjugated to tetanus toxoid vaccine's vaccine efficacies by age group were 70·6% (6·4–93·0) for children aged nine months to 2 years; 79·6% (45·8–93·9) for children aged 2–4 years; and 79·3% (63·5–89·0) for children aged 5–12 years.
These phase 3 clinical trial results, published by The Lancet on January 25, 2024, support current World Health Organization (WHO) recommendations in typhoid-endemic areas for mass campaigns among children aged nine months to 15 years, followed by routine introduction in the first two years of life.
An estimated 11–21 million cases of typhoid fever and 5 million cases of paratyphoid fever occur worldwide each year, causing an estimated 135,000–230,000 deaths. The CDC says there are very few typhoid cases in the United States each year.