Search API
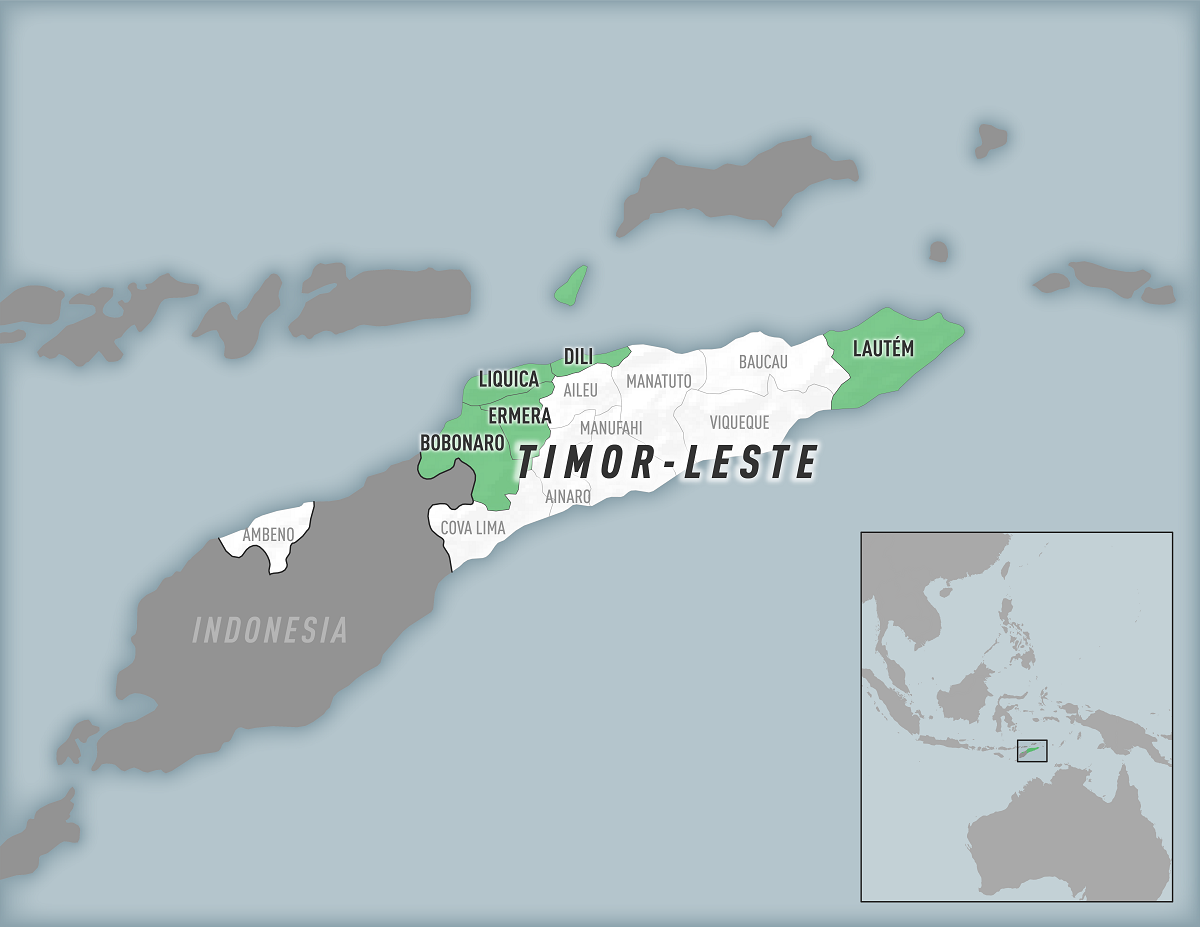
The U.S. Centers for Disease Control and Prevention (CDC) issued a Travel Health Notice today confirming a chikungunya virus outbreak is impacting several municipalities in Timor-Leste, also known as East Timor.
Located about 2,000 miles north of Australia, local media recently reported about 183 chikungunya cases.
Timor-Leste's Vice-Minister of Health previously noted that "since the Restoration of Timor-Leste's Independence, we have faced many problems, including several diseases. Being a tropical country, our country is considered an endemic zone for several infectious diseases (malaria, dengue) caused by vectors (mosquitoes)."
On February 8, 2024, the CDC said people could avoid this mosquito-transmitted disease by using insect repellent, wearing long-sleeved shirts and pants, staying in places with air conditioning, or using window and door screens.
If infected, you should seek medical care if you develop fever, joint pain, headache, muscle pain, joint swelling, or rash during or after travel.
However, chikungunya can be fatal.
A recent study published by the Lancet Infectious Diseases journal found chikungunya disease is associated with an increased risk of death for up to 84 days after symptom onset.
Furthermore, the CDC stated in this Level 2 - Practice Enhanced Precautions Notice that if you are pregnant, reconsider travel to Timore-Leste, mainly if you are close to delivering your baby. Mothers infected around the time of delivery can pass the virus to their baby before or during delivery.
Newborns infected this way or by mosquito bites are at risk for severe illness, including poor long-term outcomes.
While the U.S. FDA recently approved Valneva SE's IXCHIQ® Chikungunya Vaccine, the CDC has yet to authorize its use in the U.S.
The CDC's vaccine committee is scheduled to review this vaccine on February 28, 2024. They intend to review proposed policy options for chikungunya vaccine use among U.S. adults traveling abroad.
As of February 2024, several countries have recently confirmed chikungunya outbreaks,
As millions of residents and international travelers celebrate Carnival in Rio de Janeiro today, a mosquito-borne disease may disrupt two million people per day attending the biggest festival in the world.
As of February 9, 2024, the Federative Republic of Brazil reported over 10,000 dengue virus cases, compared to 2.9 million in 2023.
And Rio's Carnival could be a hot-spot for disease transmission.
Dengue is a vaccine-preventable disease, endemic in about 125 countries.
To reduce dengue infections, Brazil's health regulator approved the QDENGA® second-generation vaccine in 2023. Unfortunately, this dengue vaccine has not yet been widely distributed this year.
This means that in addition to festival-related headaches, many Carnival attendees may experience fever, pain behind the eyes, muscle and joint pain, and a blotchy rash.
Furthermore, on rare occasions, dengue can be fatal.
During 2023, a total of 2,050 deaths due to dengue in the Americas resulted in a case fatality rate of 0.049%.
There is good news from the local government.
On February 7, 2024, Brazil announced a National Plan for the Elimination of Socially Determined Diseases. This plan includes increasing vaccine access to prevent various diseases, including dengue.
Numerous countries are reporting measles cases as the global resurgence extends into 2024.
For example, there has been a measles outbreak in England.
The U.K. Health Security Agency (UKHSA) today announced that 118 laboratory-confirmed measles cases were confirmed in England last week, bringing the total number of measles cases to 465 since October 2023.
Of these cases, 71% (329 of 465) have been reported in the West Midlands, 13% in London, and 7% in Yorkshire and The Humber. The remaining cases were reported in other regions of England.
The majority (66%) of these cases are in unvaccinated children under the age of 10.
Dr Vanessa Saliba, UKHSA Consultant Epidemiologist, stated in a press release on February 8, 2024, "MMR vaccine uptake has been falling over the last decade with 10% of children starting school in England not protected."
"Parents should be aware that measles is a nasty illness for most children and, sadly for some, can be very serious and life-changing, but it is completely preventable."
"I strongly urge parents to take up the offer as soon as possible and protect their child now."
According to the U.S. CDC, measles is caused by a highly contagious virus that spreads through the air by direct contact with infectious droplets or by airborne spread when an infected person breathes, coughs, or sneezes.
All international travelers, including young children, should be fully vaccinated against measles, as infected people can spread measles up to four days before and after a rash.
Worldwide, there was a 64% increase in measles cases in 2023 compared to 2022.
As of 2024, the CDC maintains a global Watch-Level 1, Practice Usual Precautions, Travel Health Notice, identifying measles outbreaks in 47 countries.

The Joint Committee on Vaccination and Immunisation (JCVI) today announced its advice to the U.K. Government for the COVID-19 vaccine programme in spring 2024.
Similar to recent campaigns, the JCVI's advice issued on February 7, 2024, is to offer an updated COVID-19 vaccine to those at high risk of serious disease and who are, therefore, most likely to benefit from vaccination.
JCVI advises the following groups be offered vaccination this spring:
- Adults aged 75 years and over. COVID-19 vaccine uptake for the 2023 spring program for those 75 years and over was 67.5%.
- Residents in a care home for older adults.
- Individuals aged six months and over who are immunosuppressed. This follows updated advice in April 2023 on COVID-19 vaccination of children aged six months to 4 years in a clinical risk group.
Professor Wei Shen Lim, Chair of COVID-19 immunization on the JCVI, said in a press release, "The COVID-19 spring program will continue to focus on those at greatest risk of getting seriously ill, who will benefit the most from a further vaccine dose."
"It is important that everyone eligible takes up the offer this spring."
Utilizing a deployment cost of £25 per vaccine, the non-standard cost-effectiveness assessment for booster vaccination in spring 2024 indicated that vaccination was likely cost-effective when offered to most people over 65 within the assumptions describing the most plausible projected scenario.
In addition to mRNA vaccines, Novavax Matrix-M adjuvanted COVID-19 vaccine (Nuvaxovid) and HIPRA bivalent COVID-19 vaccine (Bimervax) may be used as a booster dose for certain persons in 2024.
As of February 8, 2024, 13 COVID-19 vaccines have been granted Emergency Use Listing by the World Health Organization. Recent additions include SKYCovione™ and CORBEVAX®.

When the U.S. government approved the first two respiratory syncytial virus (RSV) vaccines in 2023, the indication was for people 60 years and older to prevent lower respiratory tract disease (LRTD) caused by RSV.
Based on recent filings, it appears adults 50 years and older may have access to these important vaccines in time for the next RSV season.
Increasing access to RSV vaccines is viewed as a global public health goal. As of early February 2024, the percentage of U.S. adults age 60+ who report receiving an RSV vaccine this season is 20.8%.
GSK plc announced on February 6, 2024, that the U.S. Food and Drug Administration (FDA) has accepted under Priority Review an application to extend the indication of AREXVY™ adjuvanted RSV vaccine to adults aged 50-59 who are at increased risk for RSV disease.
The Prescription Drug User Fee Act date, the FDA action date for their regulatory decision, is June 7, 2024.
Previously, on December 12, 2023, Japan's Ministry of Health, Labour, and Welfare accepted a similar application for AREXVY.
If approved, AREXVY would be the first RSV vaccine available to help protect this population during the 2024-2025 RSV season.
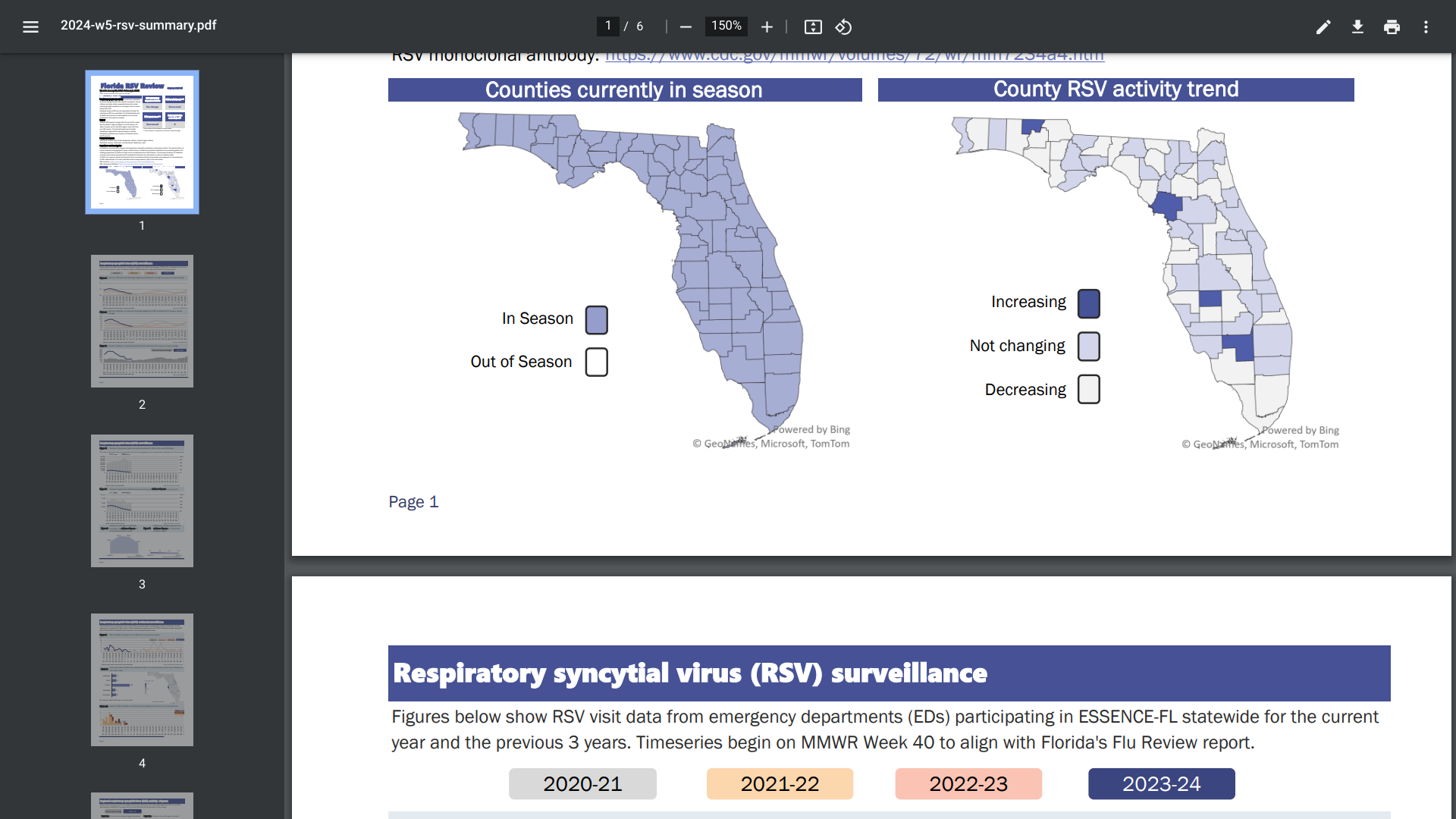
As of February 2024, RSV activity was stable or decreased in North America, and most European reporting countries reported the World Health Organization Influenza Update N° 462.
In the U.S., the Centers for Disease Control and Prevention's RSV detection graphs display the 5-week moving average, recently indicating decreased cases in certain states.
The Weekly Influenza Vaccination Dashboard uses various data sources to share preliminary weekly flu vaccination data, including coverage estimates.
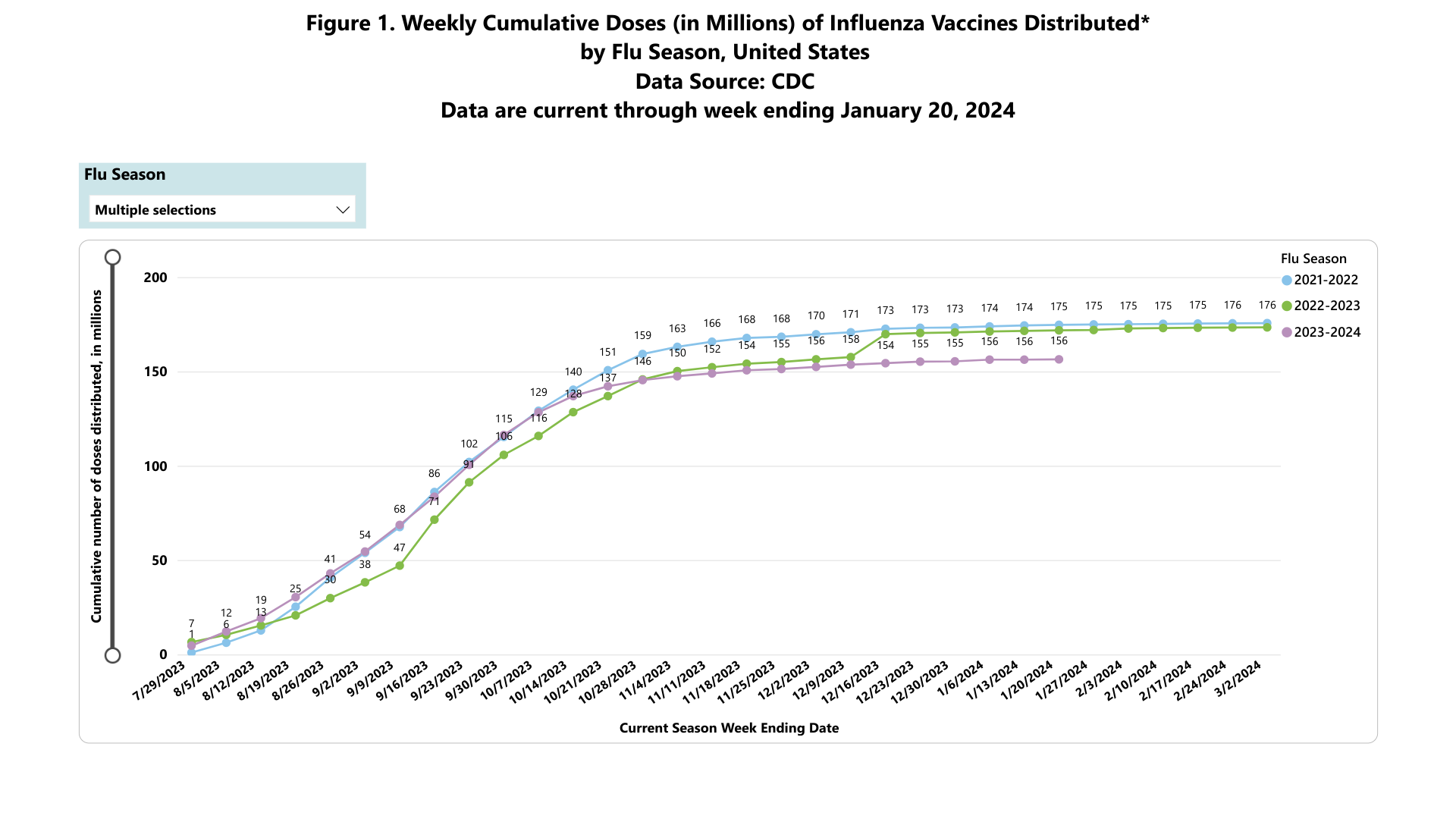
According to recent data published by the U.S. Centers for Disease Control and Prevention (CDC), influenza vaccination rates have been trending lower over the past three flu seasons.
As of January 20, 2024, the CDC's data indicates about 156 million influenza vaccines have been distributed during the 2023-2024 season. However, this data may change as the flu season progresses.
During the 2021-2022 season, 176 million vaccines were distributed in the U.S.
From a demographic perspective, the CDC reports the flu shot coverage estimates for the 2023-24 season are as follows:
Coverage for all children is 3.5% lower this season than last season (47.8% compared with 51.3%). Coverage this season so far is 9.6 percentage points lower compared with pre-pandemic coverage at the same time in January 2020 (57.4%).
For pregnant women, coverage at the end of December 2023 (35.7%) is 3.3% lower compared with coverage at the end of December 2022 (39%).
National coverage for all U.S. adults is 47%. For adults 65 years and older, an estimated 43.4% of Medicare fee-for-service beneficiaries have been vaccinated this season.
And flu shot coverage among states and D.C. ranges from 36.8% to 62.3%.
Last week, the CDC stated seasonal influenza activity remains elevated nationally, with increases in some parts of the country. And it recommends that everyone six months and older get an annual flu vaccine as long as influenza viruses are spreading.