Search API
As Lunar New Year festivities take place worldwide, there have been reports of avian influenza outbreaks in Asia and sporadic cases of human infections.
In light of this health risk, the World Health Organization (WHO) issued an updated Disease Outbreak News (DONs) on February 13, 2024, with prevention advice titled "Avian Influenza and Lunar New Year Festivities: Vigilance and Precautions" in February 2024.
During February 2024, WHO avian influenza alerts were issued for:
- Influenza A (H1N1) variant virus - Brazil, 7 February 2024
- Avian Influenza A (H5N1) - Cambodia, 8 February 2024
- Influenza A(H1N1) variant virus - Spain, 9 February 2024
- Avian Influenza A(H10N5) and Influenza A(H3N2) coinfection - China, 13 February
The WHO says vigilance remains crucial, although most human infections have been sporadic following contact with infected poultry and/or their environments, with no evidence of sustained human-to-human transmission.
Birds are the natural hosts for avian influenza viruses.
After an A(H5N1) virus outbreak in 1997 in poultry in Hong Kong SAR, China, since 2003, this avian and other influenza viruses have spread from Asia to Europe and Africa. I
Beginning in 2013, human infections with the influenza A(H7N9) virus were reported in China.
WHO DONs provide information on confirmed acute public health events or potential events of concern. For more details, please refer to the WHO Influenza (avian and other zoonotic) factsheet.
As of 2024, the U.S. government has invested tens of millions in vaccines protecting people from certain avian influenza viruses. Furthermore, the U.S. says annual flu shots are unlikely to protect people during avian influenza (bird flu) outbreaks.

As the global cholera epidemic enters another year, the World Health Organization (WHO) continues classifying cholera's resurgence as a grade 3 emergency, its highest internal level for emergencies.
And access to protective vaccines is decreasing.
On February 12, 2024, the WHO published its 11th multi-country cholera outbreak External Situation Report, which confirmed the global cholera response continues to be affected by a critical shortage of Oral Cholera Vaccines (OCV).
From January 2023 to January 2024, urgent requests for OCV surged, with 76 million OCV doses requested by 14 countries, while only 38 million doses were available during that period.
The global stockpile of cholera vaccines is awaiting replenishment, and all production up to March 8, 2024, will be allocated to approved requests.
The U.S. CDC recommends vaccination for people traveling to or living in areas of active cholera transmission. However, cholera vaccinations are not 100% effective.
Vaxchora, a single-dose, oral vaccine, is U.S. FDA-approved for use in people aged 2–64.
The WHO has approved three other OCVs.

In May 2022, when the mpox outbreak was first reported in Europe, most government health agencies started giving out Bavarian Nordic's JYNNEOS® (MVA-BN®, IMVAMUNE®) vaccine for free to eligible individuals.
As per a recent alert by the U.S. CDC COCA, only 25% of the two million eligible people have completed the JYNNEOS two-dose vaccination series in the U.S.
The CDC stated on February 12, 2024, that although the number of mpox outbreaks in the U.S. has decreased significantly since the summer of 2022, small clusters of the disease continue to occur, and severe manifestations of mpox, including deaths, are still being reported in 2024.
Furthermore, the CDC strongly urges clinicians, health departments, and community-based organizations to continue recommending the two-dose JYNNEOS vaccine to eligible individuals.
And the CDC is encouraging those who have only received one dose to get the second dose to obtain the best possible protection.
Moreover, the U.S. FDA recently approved JYNNEOS to prevent mpox infection, regardless of the mpox virus Clade.
This vaccine is generally available in major cities at health clinics and pharmacies. If you need more information about this update or wish to provide feedback, please get in touch with the CDC at [email protected].

Throughout the 2023-2024 respiratory syncytial virus (RSV) season, newly approved vaccines have been offered to pregnant women and older adults. As with all vaccines, it takes time to appreciate their ability to protect people from disease fully.
According to a report by TD Cowen's analyst Tyler Van Buren on February 8. 2024, Moderna Inc.'s mRNA-based RSV vaccine candidate may not be as effective as its competitors.
The Wall Street firm's report cites a Phase 3 clinical trial, which found that Moderna's mRNA-1345 vaccine candidate has an overall efficacy of 63.3% against two-symptom RSV disease after a follow-up of 8.6 months.
This is a significant change from a January 2023 reading, which showed mRNA-1345 had an efficacy of 84%.
"In the absence of head-to-head clinical trials, comparative conclusions regarding the safety and efficacy of mRNA-1345 relative to other RSV vaccines cannot be made," Moderna said in this abstract.
Moderna has previously confirmed it has submitted regulatory filings to the FDA for its RSV vaccine, indicating potential approvals ahead of the 2024-2025 RSV season in the U.S.
As of February 9, 2024, the U.S. CDC estimated the percentage of adults 60+ receiving an RSV vaccine was 22.4%. As of January 27, 2024, certain pregnant women's overall RSV vaccination rate was 16.2%.
As we approach Spring 2024, many individuals eagerly anticipate the end of the 2023-204 flu season. However, this flu season continues to pose a significant health risk for many people.
Globally, the World Health Organization recently released Influenza Update N° 464, indicating a decrease in influenza detections.
In North America, influenza activity is elevated, albeit declining.
The U.S. CDC reported on February 9, 2024, that seasonal influenza activity remained high across the nation, with certain regions, such as regions 5 and 7, experiencing significant increases.
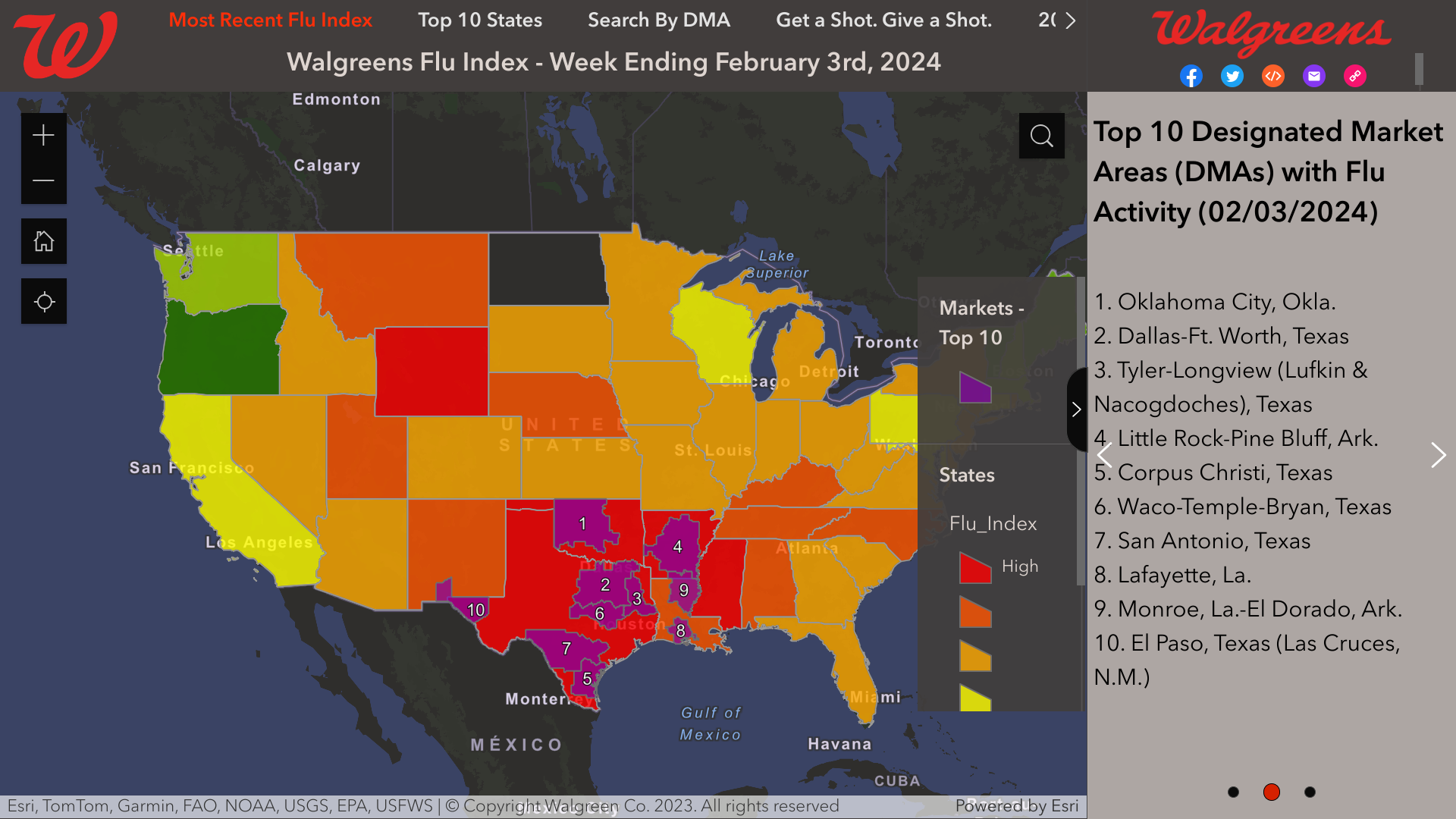
Additionally, the Walgreens Flu Index®, which provides a state and market-specific overview of flu activity, identified its top 10 cities confronting flu outbreaks as of February 3, 2024.
These cities are as follows:
- Oklahoma City
- Dallas-Ft. Worth
- Tyler-Longview
- Little Rock-Pine Bluff
- Corpus Christi
- Waco-Temple-Bryan
- San Antonio
- Lafayette
- Monroe, La.-El Dorado
- El Paso, Texas, including Las Cruces, N.M.
Note: Walgreens data is limited to the markets with its 8,700 pharmacy locations.
Unfortunately, the CDC confirmed eight influenza-associated pediatric deaths were reported during Week #5, bringing the 2023-2024 season total to 74 pediatric deaths.
The CDC continues recommending various flu shots for most people and encourages everyone to speak with a doctor, nurse, or pharmacist regarding any influenza questions.
As of late January 2024, over 157 million influenza vaccines were distributed in the U.S. this flu season.
The Maricopa County Department of Public Health (MCDPH) today confirmed a measles case involving an international visitor. This is the first measles case in 2024.
During 2021, there were 67 measles cases in Arizona.
Maricopa County, which includes the city of Phoenix, has a population of about 4.5 million.
On February 10, 2024, Dr. Nick Staab, assistant medical director for MCDPH, commented in a press release, "Measles is both highly infectious and completely preventable."
"We encourage residents to stay up-to-date on their vaccines and watch for symptoms of measles, especially if you are high-risk or unvaccinated," Dr. Staab added.
It can take up to 21 days after their last exposure for a person infected with measles to start showing symptoms.
Measles is a vaccine-preventable disease. Various measles vaccines are offered at most clinics and pharmacies in the U.S.
A CBS News investigation revealed on January 30, 2024, that at least 8,500 U.S. schools risk measles outbreaks in 2024 due to low vaccination rates. Data sources indicate about 90% of children in Arizona have been vaccinated against the measles virus.
During the first six weeks of 2024, measles cases were reported in Dayton, San Diego, Montgomery County, MD, Los Angeles, Philadelphia, Atlanta, Northern Virginia, Camden County, NJ, Kansas City, Wilmington, and Clark and Wahkiakum Washington counties.
As of January 25, 2024, the U.S. CDC reported a total of 9 measles cases were reported by four jurisdictions, mainly related to international travelers. During 2023, a total of 58 measles cases were reported by 20 jurisdictions.
In 2023, the CDC published a global Watch-Level 1, Travel Health Notice, identifying measles outbreaks in 47 countries.

The journal Eurosurveillance Rapid Communication published a study on January 25, 2024, that concluded Beyfortus™ (Nirsevimab) was about 69% effective at preventing pediatric respiratory syncytial virus (RSV) hospitalizations in infants.
In the context of moderate to high immunization coverage (84%) among neonates, this study provides early real-world evidence of nirsevimab immunization protecting infants from severe RSV disease in Luxembourg.
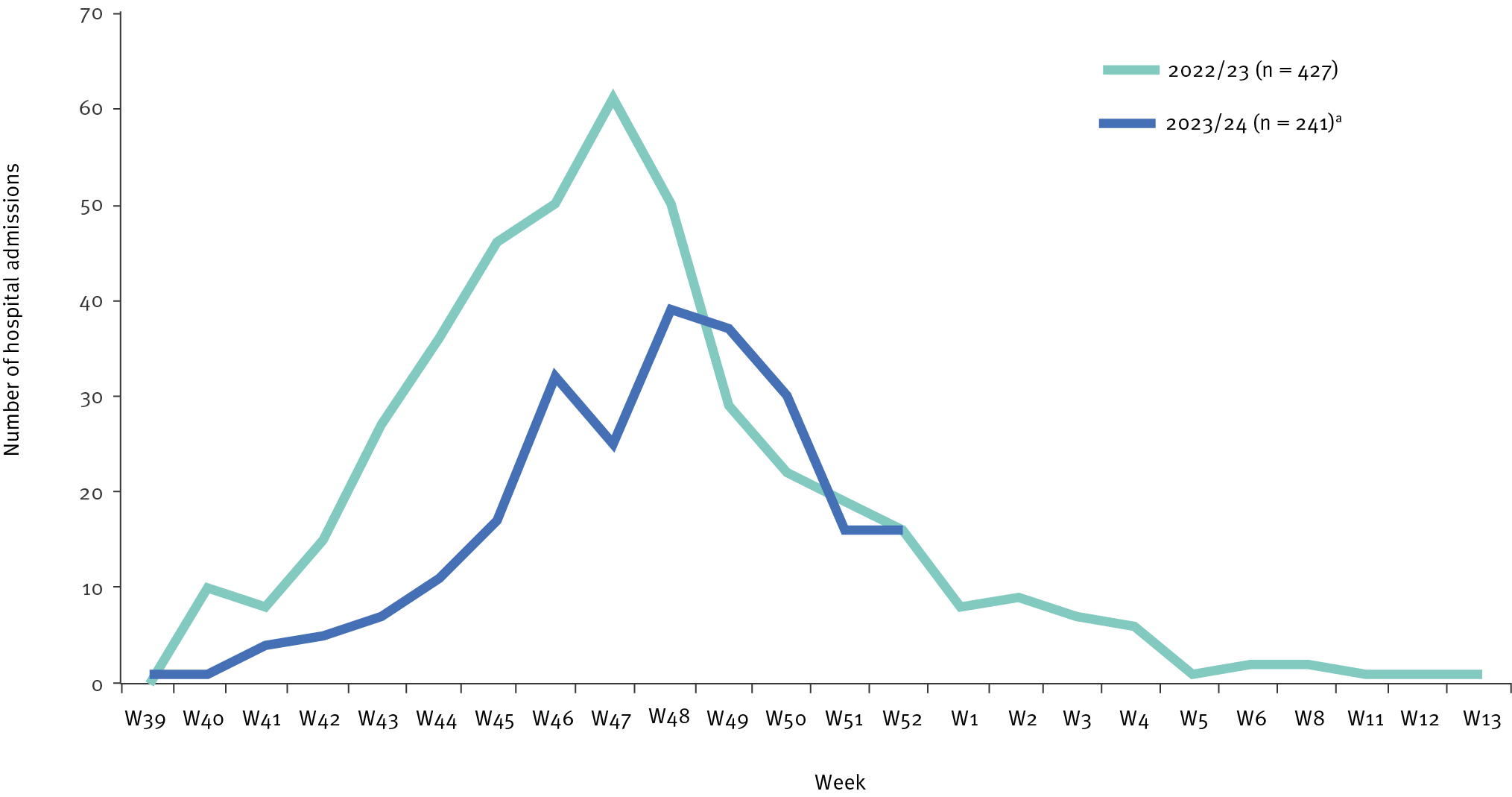
In 2023, 241 children under five years of age were hospitalized with a laboratory-confirmed RSV infection, compared with 389 cases in 2022, representing decreases of 38% (389 vs. 241) in cases under five years of age and 69% (232 vs. 72) in cases of infants under six months old.
The researchers concluded, 'Our study shows the impact of nirsevimab in mitigating severe RSV disease among infants during the first RSV season following the national implementation of passive immunization achieving high coverage in Luxembourg. There was a significant increase in the age of hospitalized children, and most severe RSV-related hospitalizations occurred in non-immunized children."
In previous clinical trials. nirsevimab showed between 74% and 86% efficacy against medically-attended lower respiratory tract infections caused by RSV in healthy infants.
As of February 2024, Beyfortus's availability in the U.S. has significantly improved.
YS Biopharma Co., Ltd. today announced that it has entered into a share purchase agreement with an institutional investor for a private placement for an aggregate of US$40 million in proceeds.
As of February 9, 2024, YS Biopharma has developed a proprietary PIKA® immunomodulating technology platform and a new generation of preventive and therapeutic biologics targeting Rabies and other virus infections.
According to the World Health Organization, Rabies is a vaccine-preventable viral disease found in more than 150 countries and territories.
The PIKA rabies vaccine candidate is a lyophilized human-use rabies vaccine composed of cell culture-derived rabies antigen mixed with PIKA adjuvant, which acts as a TLR3 agonist. It is designed to induce accelerated and strong cellular immunity and rapidly stimulate the body to produce a higher humoral immune response.
And its accelerated onset of immune response allows a three-visit, one-week regimen superior to the currently available vaccine with a five-visit, one-month or three-visit, three-week regimen, which shortens the treatment period by two to three weeks.
The clinical studies to date have shown that PIKA rabies vaccine can achieve protective level of neutralizing antibodies as early as seven days post vaccination and elicit more robust immunogenic response compared to that of the control arm vaccine, which is a widely used commercially available vaccine.
On October 31, 2023, the company completed enrollment in its Phase 3 clinical trial, which will assess the safety, immunogenicity, and lot-to-lot consistency of the PIKA Rabies Vaccine.
On June 1, 2023, the Food and Drug Administration of the Philippines granted Phase 3 clinical trial approval, and on May 16, 2023, Pakistan issued study approval.
In the U.S., dog control programs were initiated in the 1940s. Since then, routine rabies vaccination eliminated the canine rabies virus variant from circulation by 2008.
As of 2024, bats are the leading cause of rabies deaths in people in all 49 continental U.S. states. Currently, several rabies vaccines for people, such as Chirorab®, are available worldwide.