Search API
During the global measles outbreak of 2024, the UK Health Security Agency (UKHSA) announced yesterday that there has been a potential slowing of measles cases in England.
On February 15, 2024, the UKHSA reported that since October 2023, England has confirmed 521 measles cases. There were 252 in January and 50 so far in February 2024.
From a location perspective, 69% (358 of 521) of these cases have been in the West Midlands, 14% in London, and 7% in Yorkshire and The Humber. The remaining cases were reported in other regions of England.
Across the West Midlands, the population centers of Birmingham (1.1 million) and Coventry (350,000) are located.
Dr Vanessa Saliba, UKHSA Consultant Epidemiologist, commented in a press statement, "As expected, due to worryingly low MMR vaccine uptake in some areas across England."
"We are now seeing clusters of cases in other (England) regions."
There are two different brands of MMR vaccine available in the UK. These are called Priorix and MMRVaxPro.
The UKHSA stated the data published in this epidemiological overview is currently provisional, with the number of cases for the most recent months likely to increase.
According to the U.S. Centers for Disease Control and Prevention (CDC), there has been a rise in person-to-person outbreaks of hepatitis A in the United States since 2016.
On February 15, 2024, the CDC released a statement that the risk of hepatitis A transmission is higher in jails and presented a case study on reducing the risk of this disease.
The Los Angeles County Jail system was notified on May 30, 2023, that the Los Angeles County Jail system, the largest in the United States, was notified that an incarcerated person, a food handler, had received a positive hepatitis A test result.
Using electronic health records and the state immunization registry, investigators identified persons eligible for hepatitis A vaccination. There were 2,766 persons who were offered the vaccine, and 54.6% agreed to receive it.
Persons initially declined vaccination were offered a second opportunity to receive a vaccine. Incarcerated kitchen workers with undocumented vaccination history or undocumented serology who refused vaccination were removed from kitchen duties until the end of their potential incubation period.
As of October 16, 2023, no additional Hep A cases have been identified in that LA jail system.
The CDC says, 'Identifying (hep A) contacts promptly and using immunization and serology records to ensure rapid delivery of postexposure prophylactic vaccine can help prevent disease transmission during exposures among incarcerated populations.'

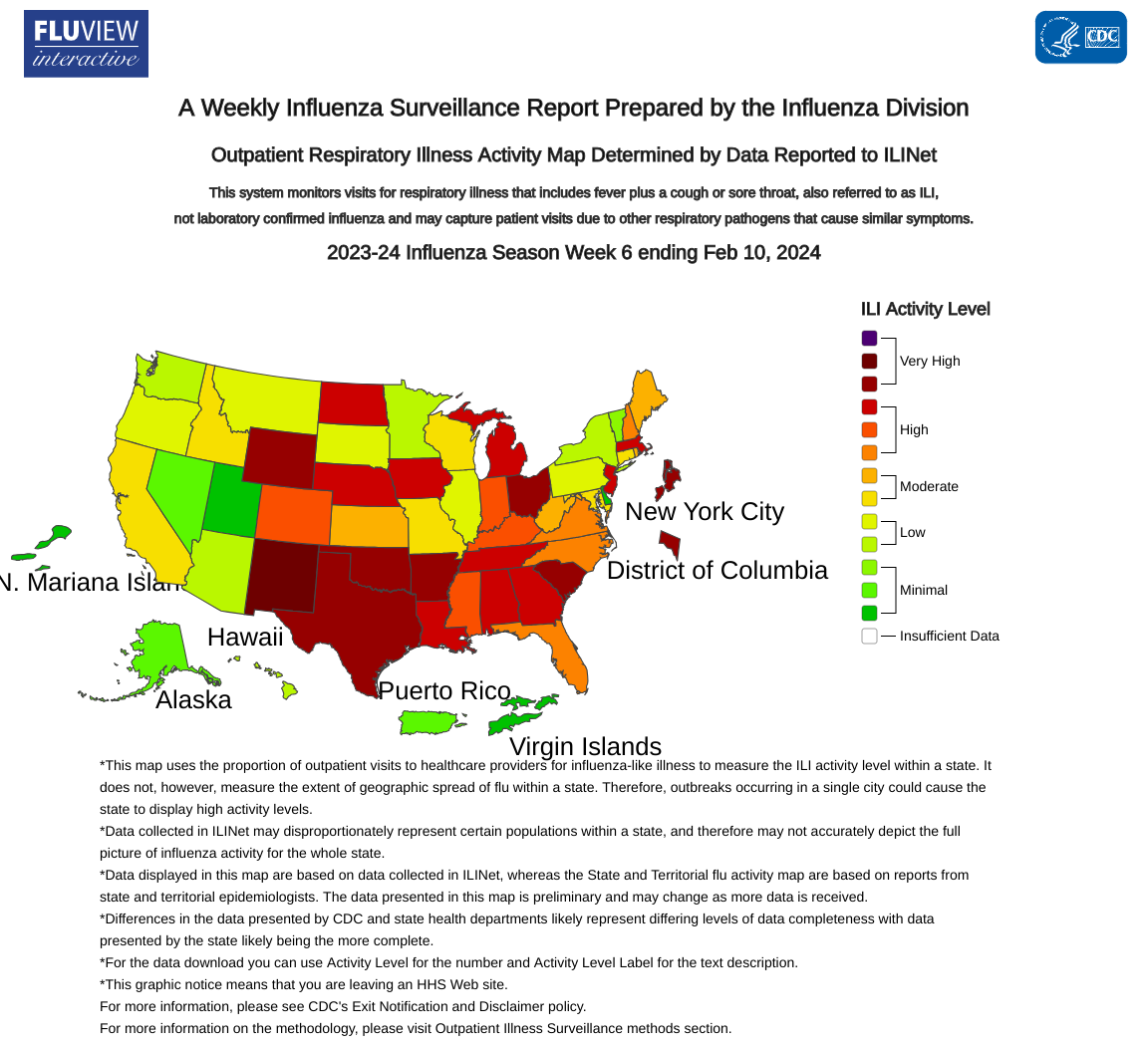
The U.S. Centers for Disease Control and Prevention (CDC) has released an update for Week #6 of the 2024 flu season.
As of February 16, 2024, the overall national percent positivity for influenza has remained stable.
However, there has been a slight decrease in percent positivity for influenza A and a slight increase in percent positivity for influenza B. This change has been primarily driven by U.S. regions 3, 5, and 7 activity.
CDC estimates that there have been at least 16,000 deaths from influenza so far this season.
During Week 6, eight influenza-associated pediatric deaths were reported, bringing the 2023-2024 flu season total to 82.
During the last flu season, there were 182 pediatric influenza-related deaths.
The CDC added vaccination can still provide benefits this season. Everyone six months and older can get an annual flu vaccine at a local pharmacy if influenza viruses spread.
As of early February 2024, about 156 million flu shots had been distributed.
A new meta-analysis published by the Lancet concluded preventive products for respiratory syncytial virus (RSV) can have a substantial public health impact by preventing RSV-severe outcomes in preterm infants.
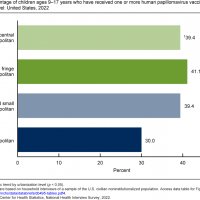
According to this article posted on February 14, 2024, preterm infants accounted for about 25% of RSV hospitalizations.
This systematic review and meta-analysis found early and late preterm infants had higher RSV-associated ALRI incidence and hospitalization rates than all infants of any gestational weeks.
And the increased risk of hospitalization among early preterm infants persisted into the second year of life.
One explanation is that early preterm children are at higher risk of underlying medical conditions with long-term impacts, such as chronic lung diseases and bronchopulmonary dysplasia, as shown by the ad-hoc exploratory analysis of the prevalence of comorbidities, wrote these researchers.
The U.S. CDC says preterm birth is when a baby is born before 37 weeks of pregnancy. In 2022, preterm birth affected about 10% of infants born in the U.S.
Approved RSV preventive products in 2024 include vaccines and monoclonal antibody (mAb) passive immunization.
In 2023, the phase 3 MELODY clinical trial assessed the efficacy of Beyfortus™ (Nirsevimab), a U.S. FDA-approved mAb, in infants born at a gestational age of at least 35 weeks.
This study found in term and late-preterm infants, a single dose of Beyfortus provided consistent protection against hospitalization for RSV-associated lower respiratory tract infection and severe, medically attended RSV-associated lower respiratory tract infection during an RSV season.

Following the end of the recent pandemic, many travelers explored new destinations in 2023. However, over 667,000 cholera cases and 4,000 deaths were reported last year.
In countries experiencing cholera outbreaks, international travelers protected themselves with safe and effective vaccines.
Since the beginning of 2023, there have been 24 reactive cholera vaccination campaigns implemented in 12 countries.
But, there is a global shortage of cholera vaccines. From January 2023 to January 2024, 76 million oral cholera vaccines (OCV) were requested by 14 countries, while only 38 million doses were available.
The World Health Organization (WHO) has three pre-qualified OCVs: Dukoral®, Shanchol™, and Euvichol®.
One of these OCV manufacturers reported a 72% increase in revenues today.
On February 15, 2024, Valneva SE reported its revenue for 2023. Last year, sales of Valneav's DUKORAL® vaccine amounted to €29.8 million (US32 million), a significant increase from the €17.3 million recorded in 2022.
This significant increase can be attributed to the recovery of the private travel markets and price hikes. However, the company says that foreign currency fluctuations caused a reduction of €0.9 million in DUKORAL® sales.
In a press release, Peter Bühler, Valneva's Chief Financial Officer, commented, "In 2023, Valneva successfully executed key strategic objectives despite a difficult economic environment.... we are entering 2024 in a good financial position to support our commercial and R&D objectives."
In the United States, access to cholera vaccines is limited.

Vaxxinity, Inc. announced today that it has published data from multiple non-human primate studies that demonstrate the VXX-401 vaccine candidate consistently reduces low-density lipoprotein cholesterol (LDL-C) in non-human primates.
VXX-401 is a synthetic peptide vaccine designed to stimulate the immune system to produce antibodies targeting proprotein convertase subtilisin/kexin type 9 (PCSK9), which reduces circulating LDL-C by inhibiting the breakdown of low-density lipoprotein receptor (LDLR).
Across three preclinical studies in cynomolgus monkeys, VXX-401 induced a strong and durable antibody response against PCSK9 and robust, sustained reduction of LDL-C over time.
Prolonged exposure with VXX-401 resulted in an average of 44% LDL-C reduction.
VXX-401 was well tolerated and did not induce any toxicity or pathology beyond mild injection site reactions.
Previous studies have demonstrated that blocking PCSK9 yields lower LDL-C levels and reduces the risk of adverse cardiovascular events.
The company says these results suggest that VXX-401 could be a safe and effective anti-PCSK9 immunotherapy.
The first-in-human Phase 1 clinical trial of VXX-401 is ongoing, with topline results expected in mid-2024.
"Vaxxinity is committed to providing scalable, accessible, game-changing solutions for worldwide heart health," said Mei Mei Hu, CEO of Vaxxinity, in a press release on February 15, 2024.
"Despite multiple approved medications for LDL-C reduction, heart disease remains the number one killer in the world. A cholesterol vaccine like VXX-401 may provide a cost-effective and widely deployable solution that could benefit hundreds of millions of people at risk."
"A well-tolerated intervention that people can start early in life and remain on for many years, lowering the cholesterol' area under the curve,' has the potential to help us win the fight against heart disease."
The publication can be found in the Journal of Lipid Research (Volume 65, Issue 2, 100497, February 2024).

During the first six weeks of 2024, various cities in the United States have reported measles cases, mainly related to unvaccinated international travelers.
On February 13, 2024, the Minnesota Department of Public Health (MDH) confirmed two measles cases had been reported this year. CBS News revealed that the first case was found in the Twin Cities metro area, and the second occurred in an unvaccinated sibling.
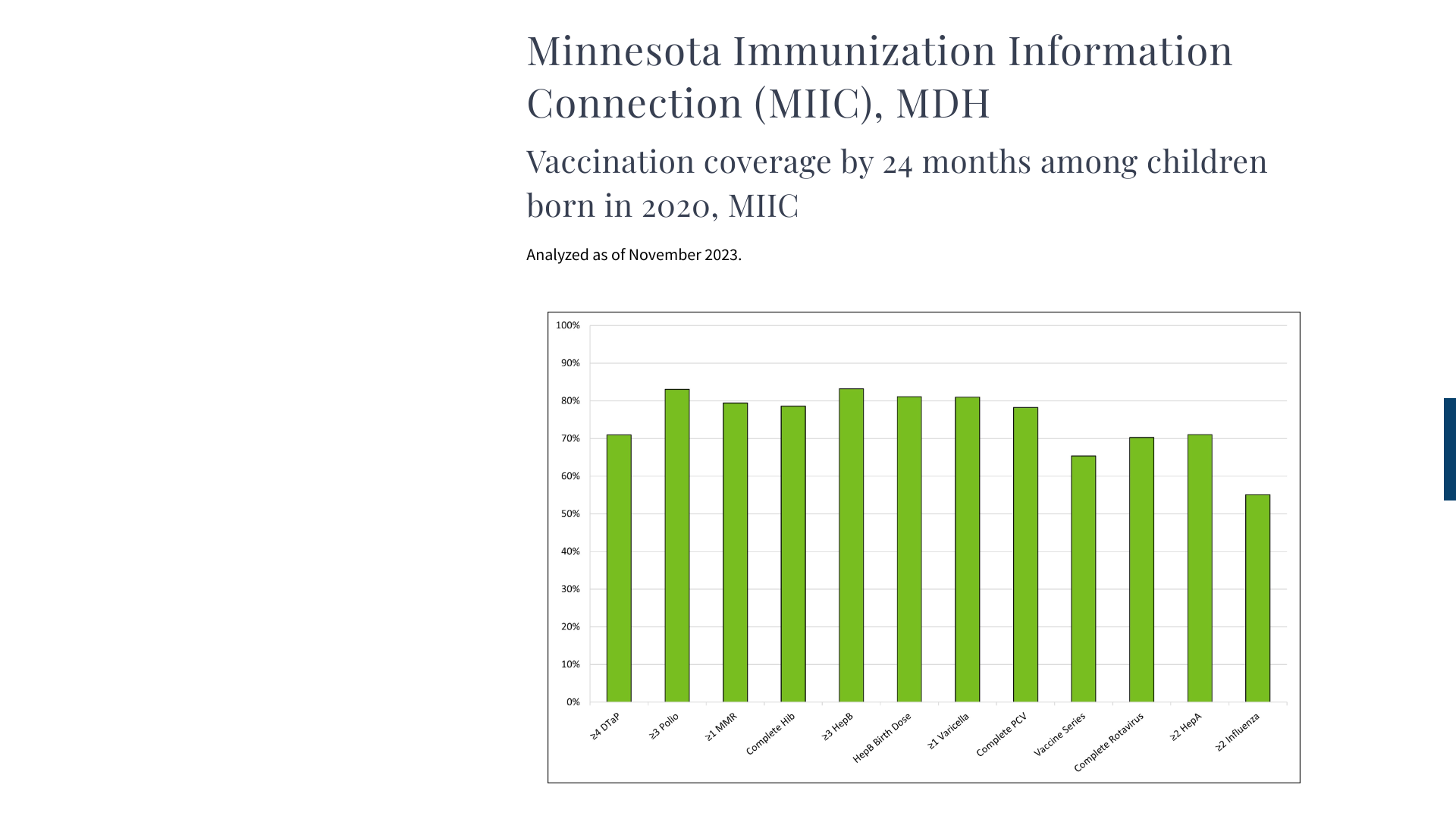
MDH says measles is a vaccine-preventable disease, but recent data indicates Minnesota is trailing the national vaccination rate.
According to MDH data, about 80% of young children were vaccinated with a measles vaccine as of November 2023. MDH reported (0) measles cases in 2023, but 22 in 2022.
Nationally, the U.S. CDC has reported a total of 9 measles cases in four jurisdictions in 2024.
Internationally, the CDC says over the past year, Yemen (18,464), Azerbaijan, Kazakhstan, and India have reported the most measles cases. In 2023, over 534,000 suspected measles cases were reported in 169 jurisdictions.
To alert travelers, the CDC maintains a global Watch-Level 1, Practice Usual Precautions, Travel Health Notice, that identifies measles outbreaks in 47 countries.