Search API
Novavax, Inc. and Gavi, the Vaccine Alliance (Gavi), today announced they have reached a settlement related to the 2021 Advance Purchase Agreement (APA) for Novavax's prototype COVID-19 vaccine.
To further a joint commitment to public health, Novavax and Gavi have agreed to terms that will prioritize Gavi's and Novavax's shared mission to save lives and protect people's health by increasing equitable and sustainable use of vaccines.
Under the terms, Novavax has made an initial payment of $75 million to Gavi and has agreed to make deferred payments of $80 million annually through December 31, 2028, which are due in quarterly installments and total up to $400 million.
Novavax's annual cash obligation would be offset or reduced pursuant to an $80 million yearly vaccine credit, which may be used for qualifying sales of any of the Company's vaccines funded by Gavi for supply to low-income and lower-middle-income countries.
Using the annual vaccine credit for qualifying sales would reduce Novavax's annual cash obligation.
In addition to the annual obligation, Novavax will provide an additional vaccine credit of up to $225 million, should there be additional demand, which can be applied towards qualifying dose purchases of any of the Company's vaccines in such countries throughout the five-year term.
"Novavax is pleased to have reached this agreement with Gavi as it gives us the ability to continue to work together toward our shared mission of ensuring equitable access to safe and effective vaccines," said John C. Jacobs, President and Chief Executive Officer, Novavax, in a press release on February 22, 2024.
"We look forward to a long-term partnership with Gavi to provide continued access to our protein-based non-mRNA COVID-19 vaccine."
This agreement brings the pending arbitration related to the APA to a close.
As of February 2024, Novavax's COVID-19 Vaccine (Nuvaxovid™XBB.1.5 dispersion for injection, NVX-CoV2601) is the leading protein-based vaccine used in various countries, including the United States.
As of February 22, 2024, Nuvaxovid is one of 13 COVID-19 vaccines Listed by the WHO.
Gavi is a public-private partnership that worked with the World Health Organization and various countries to supply vaccines during the recent pandemic.

ImmunityBio today announced that enrollment and initial follow-up have been completed for the safety portions of a phase 2b clinical trial that is studying an investigational cancer vaccine of a tri-valent combination of antigens delivered by a second-generation Adenovirus vector (Tri-Ad5 CEA/MUC1/brachyury) together with its IL-15 superagonist N-803 for participants with Lynch syndrome.
Each of the three vaccines in Tri-Ad5 targets different proteins associated with precancer and cancer cells.
The vaccine combination studies whether activating dendritic cells and training the immune system to recognize those proteins will destroy the precancer cells before the cancer occurs.
The IL-15 superagonist N-803 is designed to enhance the effects of the vaccines by increasing the proliferation and activation of natural killer and T cells, thereby increasing the potential for cancer prevention in study participants.
The study will include up to 186 participants when fully enrolled and is now open to the randomized controlled portion of the trial.
“We are pleased to be selected to participate in this important and innovative cancer prevention study, one that could provide insights into how the immune system could be harnessed to prevent cancer in individuals with hereditary risk,” said Patrick Soon-Shiong, M.D., Executive Chairman and Global Chief Scientific and Medical Officer at ImmunityBio, in a press release on February 21, 2024.
“With an estimated 5 to 10 percent of cancers inherited, understanding mechanisms that might prevent or delay their onset could potentially change the prospects for tens of thousands of people annually.”
Lynch syndrome is associated with a genetic mutation present in an estimated one million Americans who are more likely to be diagnosed with cancer at a younger age and are at increased risk of developing multiple types of cancers during their lifetime.
ImmunityBio’s Tri-Ad5 Vaccines and N-803 are investigational and are not commercially approved. The safety and efficacy of these investigational agents have not been established by any Health Authority, including the U.S. FDA.
The National Cancer Institute sponsors this study.

The global tuberculosis outbreak continues to impact millions of people in 192 countries and areas. As of 2023, the United Kingdom is no exception.
According to the annual report of UK Health Security Agency (UKHSA) on tuberculosis, there was a 10.7% increase in the number of tuberculosis cases in England in 2023, compared to 2022.
Dr Esther Robinson, Head of the TB Unit at UKHSA, said in a press release on February 15, 2024, "A cough that usually has mucus and lasts longer than three weeks can be caused by a range of other issues, including TB."
"Please speak to your GP if you think you could be at risk."
In 2022, TB notification rates varied widely across England, with the highest in London (1,575 individuals, rate of 17.9 per 100,000) and the lowest in the South West.
People with TB continue to be concentrated in large urban areas, with the highest TB notification rates being in Newham (London) and Leicester City (East Midlands).
In 2022, non-UK-born individuals continued to account for 79.1% of TB notifications in England.
People born in countries in India, Pakistan, Bangladesh, Eritrea, Nigeria, and Romania experience the highest number of cases.
The UKHSA continues to work with partners on a TB action plan, which sets out steps to improve the prevention and detection of TB.
However, the U.K. has reduced access to TB prevention vaccines.
In the United States, the U.S. CDC reported in November 2023 that TB cases increased by 5% in 2022, with 60 U.S. states, the District of Columbia, and territories provisionally reporting 8,331 TB cases.
Furthermore, about 13 million people in the U.S. are living with latent TB infection.
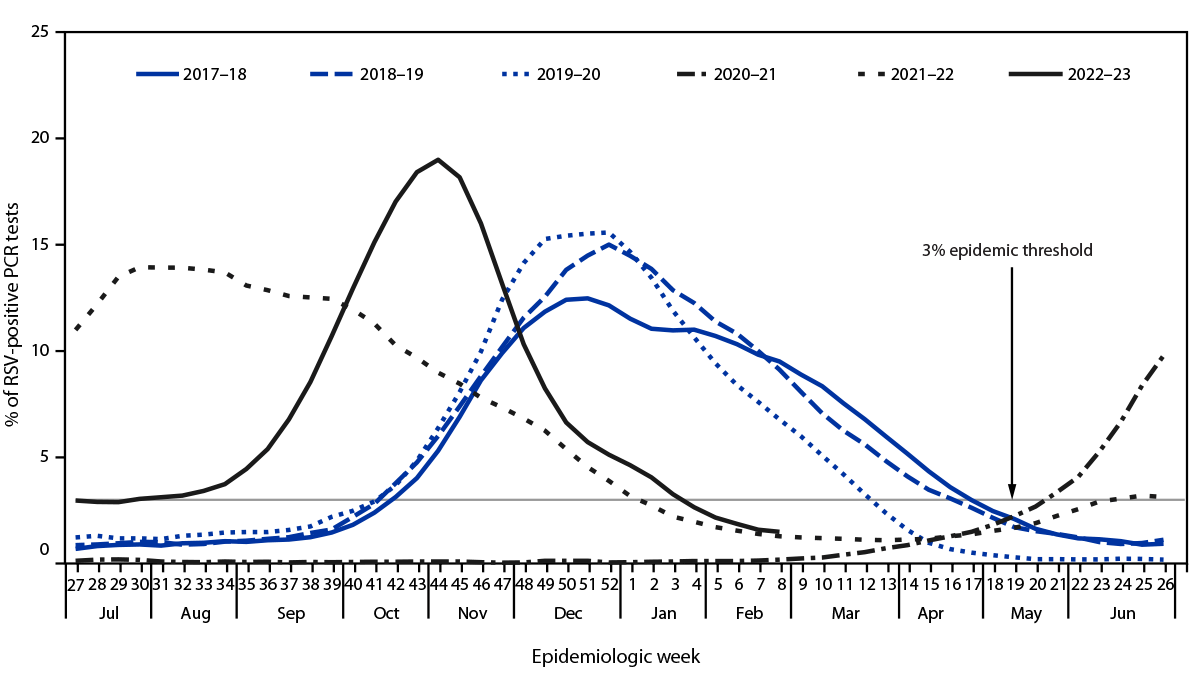
While the respiratory virus season is likely past its peak, it is not over. Respiratory syncytial virus (RSV) outbreaks exist in a few hot spots.
According to the World Health Organization (WHO) Update N° 465, in countries with RSV surveillance, activity was stable or decreased in most reporting countries except in South Africa and New South Wales (Australia), where detections slightly increased in this reporting period.
As of February 19, 2024, RSV positivity was still high in Egypt, though it slightly dropped compared with the last report.
In the United States, the Centers for Disease Control and Prevention (CDC) reported on February 16, 2024, that RSV activity remained elevated but was decreasing nationally.
Other indicators, such as emergency department visits with diagnosed RSV and RSV test positivity, are also decreasing.
From a comparison perspective, the national RSV per capita hospitalization rate remains lower than the peak for last season, says the CDC.
Furthermore, the U.S. market has ample access to approved RSV adult vaccines. At this point in the season, 22% of adults 60 years and older have received an RSV vaccination.
And a monoclonal antibody therapy (Beyfortus™) that delivers passive immunization to infants has become more available.
According to data recently reported by the U.S. Centers for Disease Control and Prevention (CDC), syphilis cases have been increasing in most states, and Alaskans are experiencing surges in this sexually transmitted disease (STD).
As of February 20, 2024, the CDC says syphilis is one STD that lacks a preventive vaccine.
"As recently as 2016, Alaska had just 20 cases of syphilis in a year. In 2022, Alaska had 424 syphilis cases or more than a twenty-fold increase," said a written statement from the Alaska Department of Health.
According to an opinion by Claudia Haines published by the Anchorage Daily News, Dr. Anne Zink, Alaska's chief medical officer, wrote, "Everyone of reproductive age who is sexually active should be tested for syphilis if they are unsure of their syphilis status."
"Everyone should get retested each time they have a new sexual partner, and every 3-6 months if they have multiple partners."
Alaska's epidemiology team identified several barriers faced by the increasing number of people testing positive for syphilis and women birthing babies with congenital syphilis.
The complete, unedited ADN news article is posted at this link.
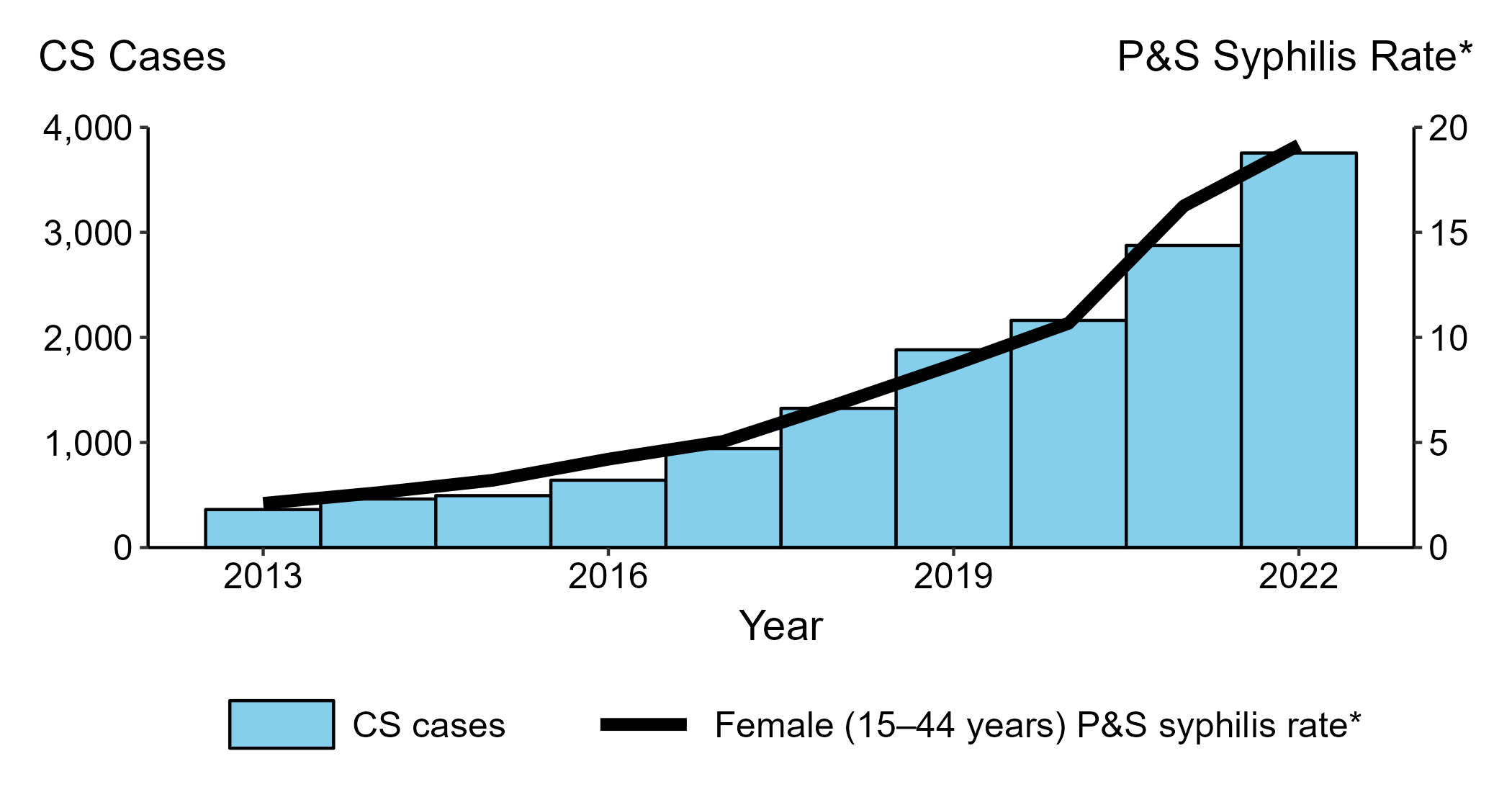
According to the CDC, the national congenital syphilis rate of 102.5 cases per 100,000 live births in 2022 represents a 30.6% increase relative to 2021. From 2021 to 2022, the rate of P&S syphilis increased by 17.2%.
In 2022, 3,755 cases of congenital syphilis were reported nationwide, including 282 congenital syphilis-related stillbirths and infant deaths.
The Florida Department of Health in Broward County (DOH-Broward) confirmed on February 19, 2024, that it is investigating multiple cases of measles at the Manatee Bay Elementary School in Weston, Florida, located just north of Miami.
A CBS News Miami article reported a 5th measles case has been confirmed at this elementary school, which has an enrollment of about 1,100 students.
According to the Manatee Bay school district, about 11% of these students are not fully vaccinated against measles.
In its press release, DOH-Broward has notified local healthcare providers, stating that those who have received the entire series of MMR vaccines are 98% protected against this airborne virus.
Measles vaccines are generally offered at health clinics and pharmacies in Florida.

During the measles outbreak of 2024, various states along the I-95 highway reported cases. Based on local news reporting, add Southeaster Florida to this expanding list.
As of February 18, 2024, Broward County health officials have confirmed four measles cases at Manatee Bay Elementary School in Weston, Florida.
News7 Miami reported John J. Sullivan, Chief Communications and Legislative Affairs Officer, Broward County Public Schools, wrote in an email, "The health, safety, and welfare of our students and staff remain our utmost priority. The District continues to work closely with the Florida Department of Health – Broward following three additional confirmed measles cases at Manatee Bay Elementary School."
As of last week, the U.S. CDC reported 20 measles cases in eleven jurisdictions. These measles cases were reported in Georgia, Maryland, New Jersey, New York City, Pennsylvania, and Northern Virginia.
Globally, the CDC says 47 countries have reported measles outbreaks during the past year.
The virus is transmitted from person to person via respiratory droplets, mainly related to unvaccinated international travelers.
Measles is a vaccine-preventable disease. Vaccines are offered throughout the U.S. at health clinics and pharmacies.
Note: This news article was updated to reflect CBS News Miami reporting on Feb. 20, 2024, a 5th measles case has been confirmed at Manatee Bay Elementary School.
The ongoing mpox outbreak in the Democratic Republic of the Congo (DRC) has reached 22 out of 26 provinces, including the capital city of Kinshasa and a few other large cities.
As of February 8, 2024, the U.S. CDC reported the DRC had confirmed 12,569 suspect mpox cases and 581 deaths since January 2023.
Furthermore, the CDC's Level 2 Travel Advisory says casual contact is not likely to cause the disease to spread. However, travelers to impacted areas should seek medical care immediately if they develop unexplained skin rash, with or without fever and chills.
The CDC says people in the United States who have already had a mpox infection or are fully vaccinated with the JYNNEOS® (MVA-BN®, IMVAMUNE®) vaccine should be protected against the type of mpox cade spreading in DRC.
JYNNEOS is a 2-dose vaccine; people need both doses for the best protection against mpox. The second dose should be given four weeks after the first dose.
In the U.S., some large cities offer JYNNEOS for free. It may be available at the health department, public health clinics, hospitals, or pharmacies.
The World Health Organization (WHO) Mpox External Situation Report #31 confirmed on December 22, 2023, that it has received mpox case reports from 115 affected countries since May 2022. Based on the data reported, the mpox outbreak will continue in most WHO regions in 2024.
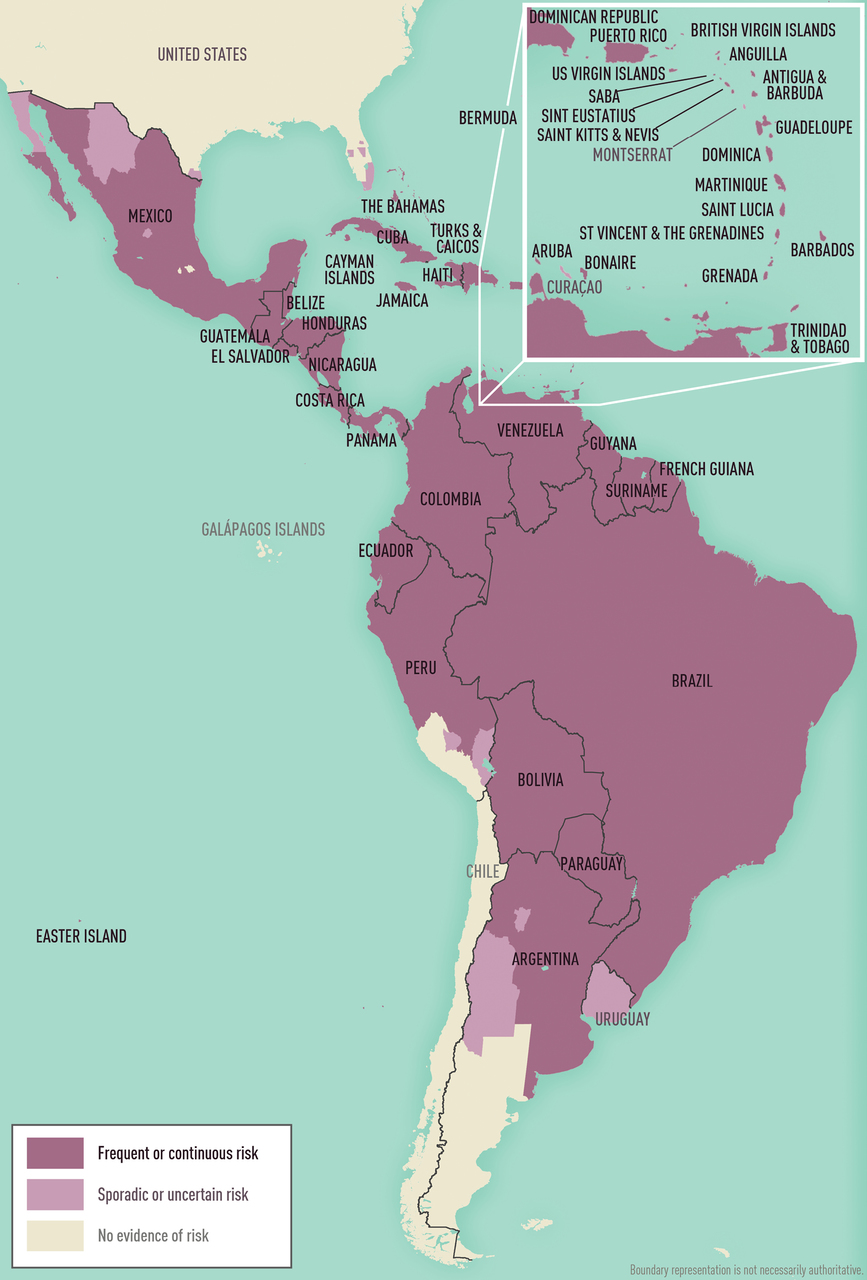
The Pan American Health Organization (PAHO) has released an Epidemiological Alert regarding the ongoing circulation of the Dengue virus in the Region of the Americas.
This region accounts for about 80% of all Dengue cases worldwide.
As of week #5 on February 17, 2024, there have been 673,267 cases of Dengue reported, out of which 102 have been fatal (case fatality rate of 0.015%). This marks a 157% increase in the number of cases from the same period in early 2023.
In 2023, a total of 4,565,911 cases of Dengue were confirmed, including 2,340 deaths.
According to the U.S. CDC, Dengue is an acute febrile illness caused by infection with any of four related single-stranded RNA viruses of the genus Flavivirus. Almost all Dengue virus transmission occurs through the bite of infected Aedes species mosquitoes.
To alert international travelers, the CDC issued Level 1 Travel Health Notices regarding dengue outbreaks in the Americas in February 2024.
From a prevention perspective, the CDC's Advisory Committee on Immunization Practices recommended the Dengvaxia® vaccine in 2021 for children aged 9–16 years with laboratory-confirmed previous dengue infection who are living in areas of the United States where Dengue is endemic.
As of February 19, 2023, the second generation QDENGA® vaccine is not authorized for use in the U.S.