Search API
While the global dengue outbreak continues in 2024, many parts of the Americas are at risk. A trendy vacation destination in Central America reports a significant surge in dengue cases this year.
According to the Costa Rica Ministry of Health, there have been 4,787 confirmed cases of dengue during the first six reporting periods of this year.
As of February 23, 2024, Costa Rica's Central North region concentrates the highest accumulated notification of dengue cases this year with 1,228 cases, followed by the Central Pacific with 832 and the Central South with 762 cases.
During 2023, there were over 24,914 dengue cases, an increase from the 7,485 dengue patients in 2022.
To alert international travelers of this mosquito-transmitted health risk, Costa Rica was included in the U.S. CDC's Level 1 - Practice Usual Precautions, dengue notice on February 09, 2024.
The Ministry of Health has called on the population to constantly clean and empty the containers in which water is stored for domestic use. Likewise, residents are urged to collaborate with our officials when they visit your homes for fumigation, presenting proper identification.
The Vector Control Program team recently carried out 16,645 fumigations in the towns of La Carpio, Pavas, and Alajuela.
As of 2024, the Dengvaxia® vaccine was sold in private pharmacies in Costa Rica. The price is around $130.
However, the second-generation QDENGA® dengue vaccine is not offered in Costa Rica.
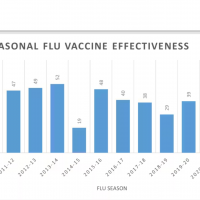
The World Health Organization (WHO) has revealed its recommended composition for influenza vaccines for the northern hemisphere flu season of 2024-2025.
Both trivalent and quadrivalent vaccines are recommended as of February 23, 2024.
These WHO recommendations are utilized by national vaccine regulatory agencies and pharmaceutical companies to develop, produce, and license influenza vaccines for the following influenza season.
Previously, the WHO urged manufacturers to eliminate the B/Yamagata component from flu vaccines for 2024-2025.
As of February 10, 2024, over 157 million flu vaccines (egg, cell, and nasal) had been distributed in the United States during the 2023-2024 season.

Bavarian Nordic A/S, a leading pharmaceutical company, announced news today regarding its investigational chikungunya vaccine, CHIKV VLP.
The European Medicines Agency's Committee for Medicinal Products for Human Use (CHMP) has granted accelerated assessment for this chikungunya vaccine candidate's Marketing Authorisation Application (MAA).
The CHMP has recognized that the vaccine candidate is of significant interest to public health and therapeutic innovation.
With this positive development, the company is taking steps toward addressing the unmet medical needs of millions worldwide affected by the Chikungunya virus.
Chikungunya outbreaks continue to be reported in 2024.
Before 2013, chikungunya virus cases and outbreaks had been identified in countries in Africa, Asia, Europe, and the Indian and Pacific Oceans. In late 2013, the first local transmission of chikungunya virus in the Americas was identified in Caribbean countries and territories, according to the U.S. CDC.
Bavarian Nordic also confirmed on February 23, 2024, that it is on track to submit its MAA for CHIKV VLP to the EMA during H1 2024. As a result, the review of the MAA may now take as little as 150 days instead of the usual 210 days.
This means that the vaccine could be available in Europe sooner than expected.
"We are pleased to receive the accelerated assessment in recognition of our chikungunya vaccine candidate and our efforts to bring this novel product to the market. With this, we can accelerate the approval and launch timelines for the vaccine in Europe. As part of our global strategy, we also plan to submit our biologics license application (BLA) for the vaccine candidate to the U.S. Food and Drug Administration later this year," said Paul Chaplin, President and CEO of Bavarian Nordic, in a press release.
In 2023, Bavarian Nordic successfully completed two Phase 3 studies of CHIKV VLP.
The CHKV-VLA vaccine candidate received the U.S. Food and Drug Administration (FDA) Fast Track designation in May 2018.
Recently, the FDA issued approval for Valneva SE's IXCHIQ® Chikungunya Vaccine. However, the CDC has not given its approval.
The UK Health Security Agency (UKHSA) today reported an additional 60 laboratory-confirmed measles cases have been confirmed in England during the ongoing outbreak.
This brings the total number of measles cases since October 2023 to 581.
As of February 22, 2024, the West Midlands accounted for most of these (47%, 79 of 169) measles cases, mainly in Birmingham, the largest city in the West Midlands, with over 1.1 million residents.
Furthermore, the majority (379 of 581, 65%) of these cases are in children.
In a press release, Dr. Vanessa Saliba, UKHSA Consultant Epidemiologist, said, "We're urging parents to protect their children from this serious illness with the MMR vaccine before it spreads further."
"...but 100s of thousands of unvaccinated children are at risk of severe illness or life-long complications."
"The disease spreads very quickly among those who are unvaccinated, especially in schools and nurseries. However, measles is entirely preventable with vaccination."
In the United Kingdom, two MMR vaccine brands are available: Priorix and MMRVaxPro.
The data published in this UKHSA epidemiological overview is currently provisional.
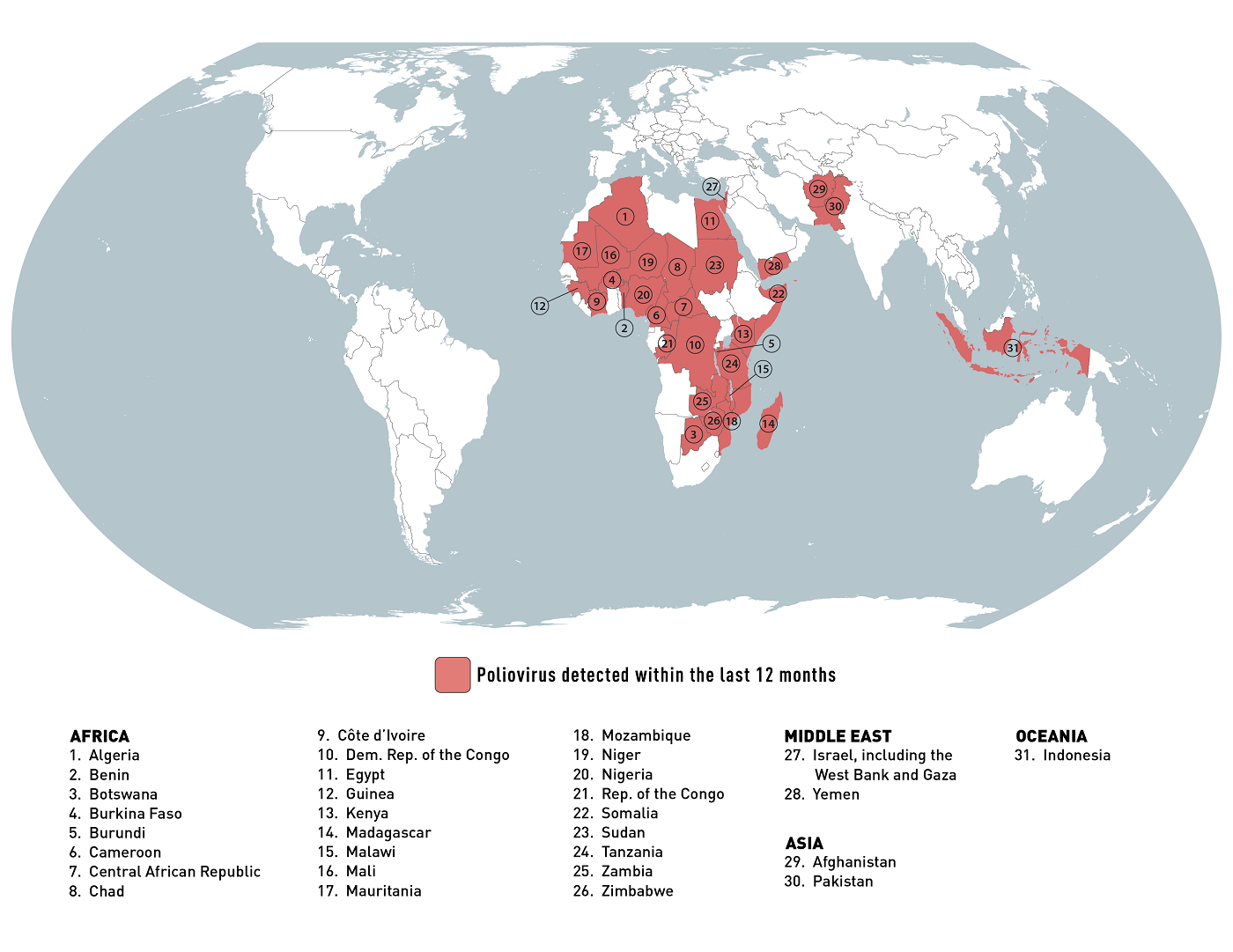
According to the AP, Zimbabwe has initiated a polio vaccination campaign to protect over four million children after identifying three polio cases caused by a rare mutation of the weakened virus found in an older version of the oral vaccine.
On February 20, 2024, the authorities confirmed they would use the type 2 novel oral polio vaccine (nOPV2) polio vaccine designed to minimize virus mutations and reduce polio outbreaks.
Zimbabwe aims to provide this vaccine to approximately 4 million children in February and March 2024.
About 1.4 billion nOPV2 doses have been produced since March 2021.
Since its launch, approximately 1 billion nOPV2 doses have been administered in more than 35 countries worldwide.
As of February 2024, the nOPV2 has not been authorized by the U.S. Food and Drug Administration. However, the U.S. CDC's vaccine committee is reviewing its use case on February 28, 2024.
Zimbabwe is one of 31 countries the CDC lists in its January 2024 global polio outbreak travel alert.
Moderna Inc. today reported $2.8 billion in Spikevax® vaccine sales in the fourth quarter of 2023. The majority of Spikevax sales ($2 billion) were in international sales.
For all of 2023, Spikevax generated $6.7 billion in vaccine sales.
Moderna confirmed in a press release on February 24, 2024, that it achieved 48% cumulative market share in the U.S. retail segment during the fall 2023 COVID season, up from 37% in 2022.
The Company reaffirmed its 2024 product sales outlook as it entered the second year of the U.S. commercial endemic COVID market.
Moderna is also prioritizing key international markets for greater commercial focus and is participating in the EU Health Emergency and Response Authority's tendering procedure for up to 36 million doses of mRNA COVID-19 vaccines per year for up to four years.
As of February 2024, Spikevax is one of 13 COVID-19 vaccines Listed by the World Health Organization.
"2023 was a year of transition for Moderna as we adapted to the endemic market. At the same time, our development team made significant pipeline advancements across infectious diseases, oncology, and rare diseases, while our commercial team increased our COVID-19 market share in the U.S.," said Stéphane Bancel, Chief Executive Officer of Moderna, in a press release.