Search API
Throughout the global measles outbreak in 2024, about 49 countries have reported cases.
Unfortunately, the United Kingdom is rapidly becoming a leader.
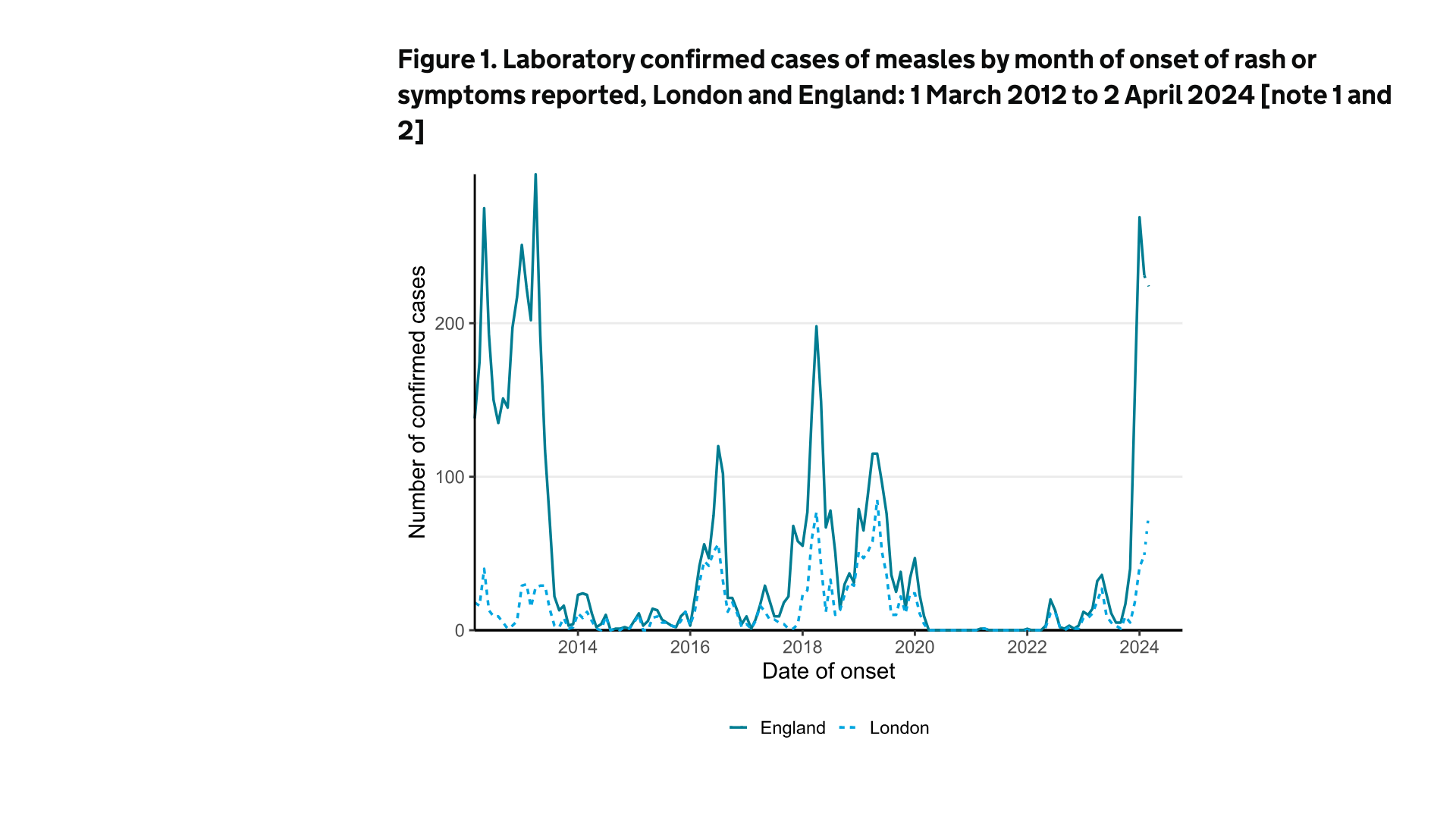
On April 4, 2024, the U.K. Health Security Agency (UKHSA) reported 269 measles cases in January 2024, 231 in February, and 224 (to date) in March 2024.
In total, there have been 934 measles cases since October 2023.
About 54% of these cases have been in the West Midlands and 21% in London.
Dr. Vanessa Saliba, UKHSA Consultant Epidemiologist, offered these insights in a press release, "We are continuing to see measles cases in all regions of England, with cases particularly high in the West Midlands and London, so it is vital that two doses of the MMR vaccine fully protect people."
"It only takes one case to get into a community with low vaccination rates for measles to spread rapidly, especially in schools and nurseries."
"We know that hundreds of thousands of children around the country, particularly in some inner-city areas, are still not fully vaccinated and are at risk of serious illness or life-long complications, but measles is completely preventable with vaccination."
Worldwide, the U.S. Centers for Disease Control and Prevention listed the top ten international measles outbreaks as of March 12, 2024, led by Kazakhstan (21,740), followed by Azerbaijan, Yemen, and India.
To alert international travelers, the CDC republished a global Watch-Level 1, Practice Usual Precautions, Travel Health Notice on March 22, 2024, identifying measles outbreaks in numerous countries.
In the United States, Chicago, Illinois, continues leading the measles outbreak in 2024.
As of April 3, 2024, Illinois has reported 56 measles cases this year, including the Chicago Department of Public Health (53) measles cases.
In the U.S., measles vaccines are generally available at clinics and community pharmacies.
Morris & Dickson today announced it became the first U.S. commercial distributor of the JYNNEOS® vaccine. This second-generation vaccine is U.S. FDA-approved to prevent mpox and smallpox disease.
On April 3, 2024, Morris & Dickson confirmed receiving the first U.S. shipment of JYNNEOS vaccines.
This vaccine must be safely stored at minus 20 degrees Celsius. Morris & Dickson's state-of-the-art distribution techniques feature this storage and transport capability.
"We are proud to be a key partner in expanding access to this first-to-market vaccine," says Layne Martin, CEO of Head of Specialty at Morris & Dickson, in a press release.
"JYNNEOS meets a critical public health need and helps ensure equitable access to healthcare, which in turn helps significantly prevent the spread of mpox to at-risk populations."
As of April 2024, U.S. healthcare providers in the U.S. can order JYNNEOS to make it available for at-risk individuals at local pharmacies and physician offices in addition to public health clinics.
In 2023, the U.S. CDC confirmed that the effectiveness of the JYNNEOS vaccine against mpox ranges from 36% to 75% after one dose and 66% to 89% for two doses. As of 2024, third-dose boosters have not been clinically approved.
Founded in 1841, Morris & Dickson is now approaching $6 billion in annual sales, making it the industry's largest independently owned full-line distributor. Independent pharmacies in the U.S. rely on Morris & Dickson for quick responses and straightforward business practices.
Since the global mpox outbreak began in early May 2022, more than 32,000 cases have been reported in the U.S., representing a third of all cases reported worldwide.
Bavarian Nordic's JYNNEOS (MVA-BN®, IMVAMUNE®) vaccine is available in various countries.

As influenza vaccine producers prepare for the 2024 - 2025 flu season, innovative vaccine candidates are progressing in clinical research, focused on enhancing efficacy and safety.
CureVac N.V. today announced interim data from an ongoing Phase 2 study, which is part of the combined Phase 1/2 study of its seasonal influenza vaccine candidate.
The purpose of this clinical trial (NCT05823974) is to find and confirm the dose and asses the reactogenicity, safety, and immune response of GlaxoSmithKline's (GSK) messenger RNA (mRNA)-based multivalent seasonal influenza vaccine (GSK4382276A) candidates administered in healthy younger and older adults.
Results from the planned interim analysis showed that the multivalent vaccine candidate using CureVac's proprietary second-generation mRNA backbone boosted antibody titers against all encoded flu strains and across all age groups and tested dose levels, including the lowest tested dose.
"The Phase 2 interim data show that CureVac's highly effective and flexible mRNA technology platform puts us on the right track to advance our joint seasonal influenza vaccine program," said Dr. Myriam Mendila, Chief Development Officer of CureVac, in a press release on April 4, 2024.
"Results regarding influenza A strains were strong. Immunogenicity for B strains was also in line with our expectations in view of other initial mRNA-based clinical flu development programs."
"We are confident that planned optimizations will improve performance against these historically challenging influenza strains."
The multivalent candidate was selected from a comprehensive Phase 1 part, which tested vaccine candidates with up to eight separate mRNA constructs per candidate.
It was designed for broad antigen coverage, encoding antigens that matched all World Health Organization (WHO) recommended flu strains.
The WHO says flu shot campaigns should be timed according to local conditions.
Countries are encouraged to analyze local surveillance information to assess their seasonality pattern at both national and subnational levels, as appropriate, to make evidence-based decisions on the timing of vaccination campaigns.
An advanced vaccine candidate for the Lassa fever virus (LASV) today announced the start of its second phase of clinical trials.
This is a significant development, as no approved vaccines for LASV are currently available.
The trial sponsor, International AIDS Vaccine Initiative (IAVI), a nonprofit scientific research organization, confirmed in a press release on April 4, 2024, that participants at HJF Medical Research International in Nigeria had been vaccinated in the first Phase 2 clinical trial of the vaccine candidate rVSV∆G-LASV-GPC.
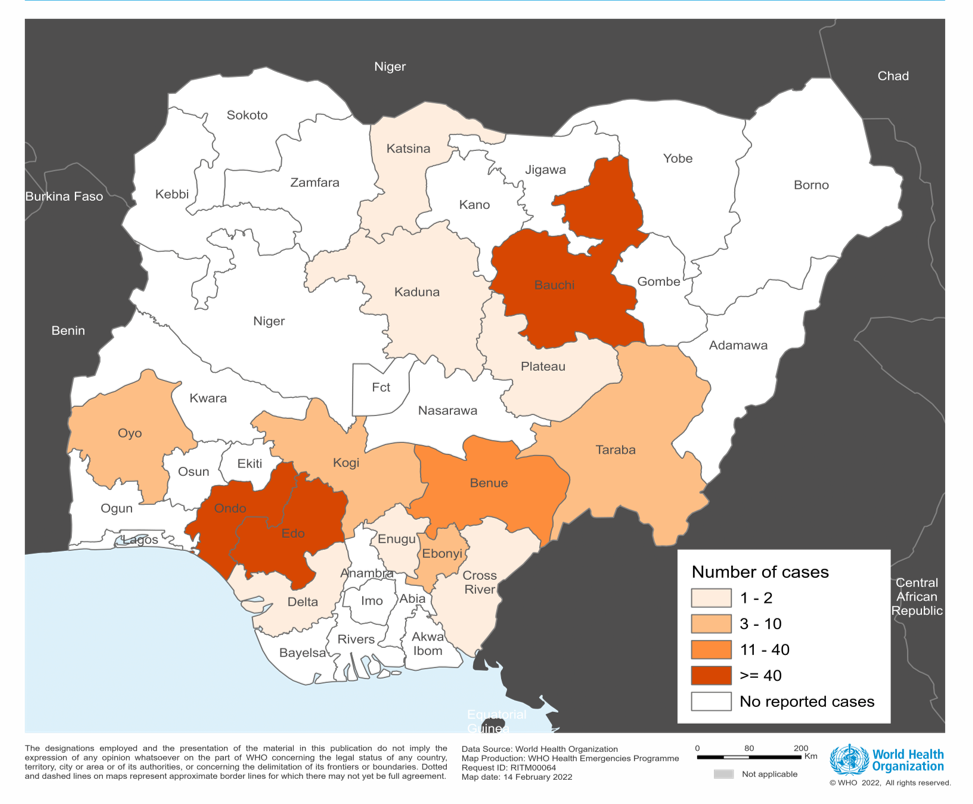
The IAVI C105/PREVAIL15 study began on March 4, 2024, and is expected to enroll over 600 people in Nigeria, Ghana, and Liberia, with results expected in 2025.
As of March 2024, 27 states in Nigeria have reported at least one confirmed case of LASV.
The vaccine has been developing since 2018 and has been supported and funded by CEPI and the European & Developing Countries Clinical Trials Partnership.
According to IAVI, rVSV∆G-LASV-GPC uses the same recombinant vesicular stomatitis virus vector platform as ERVEBO®, the single-dose Zaire ebolavirus vaccine licensed in North America, Europe, and various African countries.
“Continued outbreaks of Lassa fever and the emergence of Ebola Sudan in Uganda both underscore the need to have vaccines for known disease threats available for evaluation and use during outbreak situations – the overarching goal of IAVI’s emerging infectious disease program,” stated Swati Gupta, DrPH, MPH, vice president and head of emerging infectious diseases and epidemiology, IAVI.
The virus causes acute viral hemorrhagic illness and results in approximately 5,000 deaths and 300,000 illnesses in West Africa each year.
Furthermore, LASV has been included in the World Health Organization's R&D Blueprint of priority pathogens for which accelerated research and development and countermeasures are urgently needed.
In addition to rVSV∆G-LASV-GPC, several other LASV vaccine candidates are conducting clinical research in 2024.
As of April 2024, it remains unclear how long the immune response from mpox vaccination lasts and whether prior smallpox vaccination impacts it.
A recent study assessed the level of antibodies one year after vaccination with JYNNEOS® (MVA-BN®, IMVAMUNE®).
Announced at the European Congress of Clinical Microbiology and Infectious Diseases on March 30, 2024, this abstract indicates that people who had received smallpox vaccination during childhood and had pre-existing immunity showed high levels of antibodies generated by mpox vaccine, which remained high in almost all cases.
The authors suggested in a press release that the decrease in antibodies over time following MVA-BN vaccination may be attributable to its composition.
They stated, "The first and second-generation smallpox vaccines contained replication-competent vaccinia virus. MVA-BN is based on non-replicating virus, which may impact the strength and duration of the immune response, with the advantage of a low risk of side effects."
They add, "Regarding the potential necessity for a booster, it is premature to draw such conclusions. It is unclear how waning antibody levels relate to protection. Immunity also involves other elements, such as T-cell responses."
"Comprehensive clinical monitoring over time, which connects infection rates with antibody levels, is required to make informed decisions about booster vaccination protocols."
Bavarian Nordic A/S, the producer of JYNNEOS, the only FDA-approved mpox vaccine, recently announced that the mpox vaccine is commercially available in the U.S. at clinics and pharmacies.
The standard U.S. FDA regimen for JYNNEOS involves a subcutaneous administration with two injections of 0.5mL four weeks apart.
Note: The study is presented by Ph.D. student Dr. Marc Shamier, Erasmus MC, Rotterdam, Netherlands, from a research team led by Dr. Rory de Vries. No industry conflicts of interest were disclosed.

As polio eradication campaigns are highlighted worldwide, a new collaboration intends to support India's effort to create a polio-free country.
Bharat Biotech, the largest manufacturer of oral polio vaccines, and Bilthoven Biologicals B.V. (BBio), a wholly owned subsidiary of Serum Institute of India Private Limited, announced a collaboration to strengthen the production and supply security of Oral Polio Vaccines (OPV) further.
Through this collaboration, confirmed on April 2, 2024, BBIL and BBio will jointly obtain the regulatory approvals and licenses required to commercially manufacture OPVs in India for global supplies from drug substances manufactured in the Netherlands at BBio.
Dr. Krishna Ella, Executive Chairman of Bharat Biotech, commented in a press release, "Oral polio vaccines have been an integral part of the Govt of India's Universal Immunisation Program for several decades, with Bharat Biotech being one of the largest suppliers to immunization programs across the world."
"This collaboration between BBIL and BBio exemplifies cooperation between vaccine companies, ensuring a secure supply of oral polio vaccines and fortifies the nation's mission to eradicate polio."
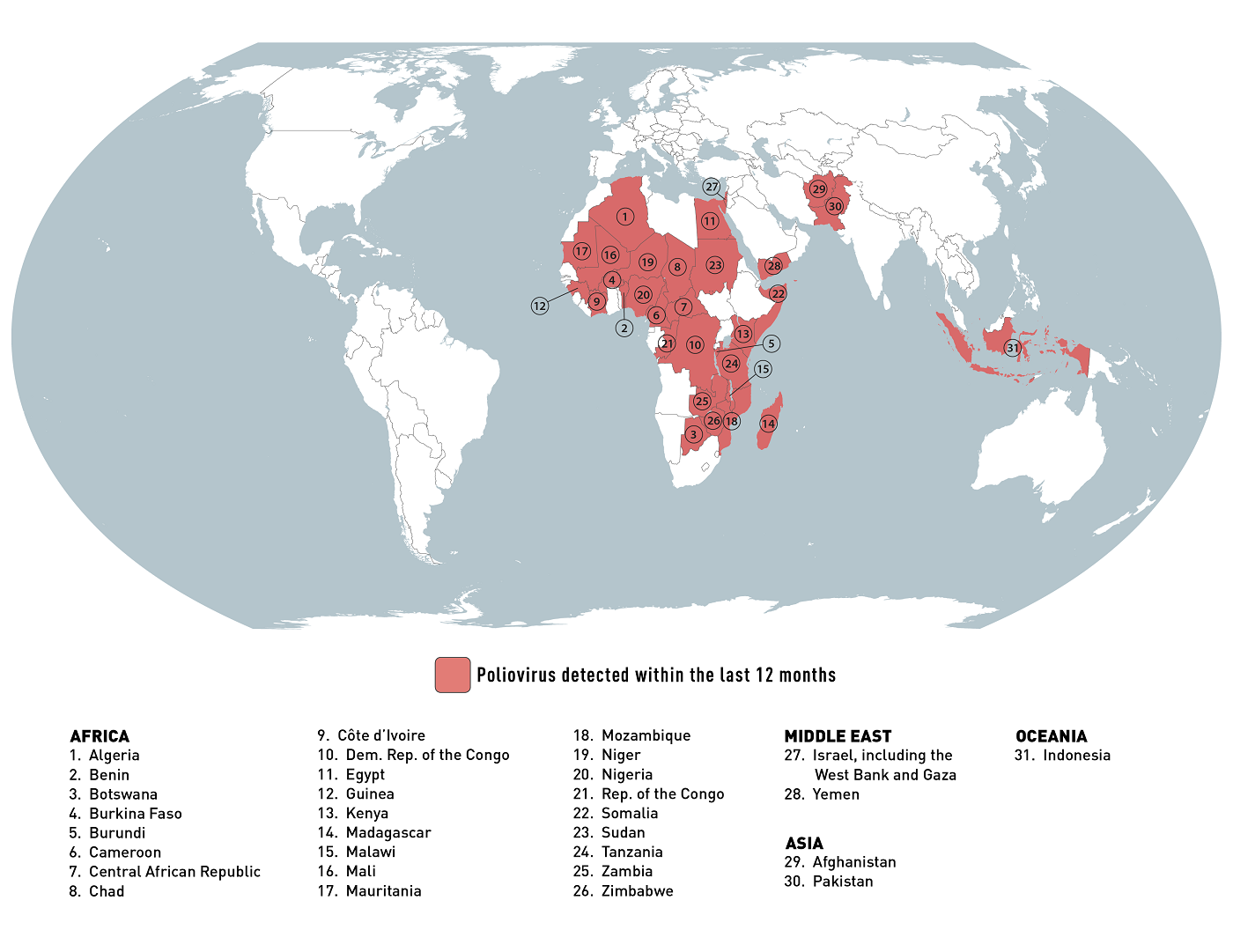
According to the U.S. CDC's Global Polio Travel Health Advisory issued in January 2024, about 31 international destinations are at risk for circulating poliovirus.
The CDC says that before visiting any at-risk destination, adults who have previously completed the full, routine polio vaccine series may receive a single, lifetime booster dose of the polio vaccine.
In the U.S., polio vaccines are offered at clinics and community pharmacies.
Longhorn Vaccines and Diagnostics today announced it is presenting a poster at the World Vaccine Congress on a mice study that examined LHNVD-110, a novel, unconjugated single peptide vaccine candidate comprised of multiple epitopes to broadly target human and influenza viruses.
According to the Company's press release on April 2, 2024, results from the poster show that LHNVD-110 generated broadly reactive antibodies to human and highly pathogenic avian influenza (HAPI) viruses while neutralizing seasonal and pandemic influenza strains.
The Company says using a single peptide provides a more cost-effective and easily scalable approach to such a universal influenza vaccine.
In this study, Longhorn examined mice that were immunized intramuscularly with a low dose (2 µg) and high dose (20 or 40 µg) of LHNVD-110, an unconjugated composite influenza peptide vaccine with multiple highly conserved epitopes of HA, NA, and matrix (M1/M2/M2e), including a universal T cell epitope.
Two booster immunizations were given on days 21 and 35. Isotype specific IgG titers to composite peptides, individual epitopes, and multiple strains of influenza A (H1N1, H3N2, H5N1), and B (Yamagata, Victoria) were analyzed by an enzyme-linked immunosorbent assay, known as ELISA.
This data mirrors previous studies with LHNVD-105, a dual peptide vaccine containing the same epitopes.
"We are studying a single peptide universal influenza vaccine because we believe it could deliver a cost-effective strategy towards formulation and manufacturing and provide immunity against human and avian influenzas," said Longhorn Vaccines and Diagnostics CEO Gerald W. Fischer, MD, in a press release.
"We are expediting IND-enabling studies of LHNVD-110 to prepare for human clinical trials."
The Company wrote, 'While traditional influenza vaccines protect against specific strains predicted for circulation during the upcoming flu season, this often leads to mismatches and variable effectiveness. That is why peptide-based vaccines with broad strain coverage may offer useful strategies for preventing influenza, no matter the strain.'
LHNVD-110 is also formulated with the AddaVax™ adjuvant from InvivoGen.