Search API
The World Health Organization (WHO) Disease Outbreak News confirmed the mpox outbreak in South Africa has expanded.
The sudden appearance of unlinked mpox cases in South Africa without a history of international travel, the high HIV prevalence among confirmed cases, and the high case-fatality ratio suggest that community transmission of the mpox virus is underway
As of July 9, 2024, 20 confirmed mpox cases, with three related fatalities in Gauteng, Western Cape, and KwaZulu-Natalhave provinces, have been reported since May 2024.
These mpox cases are South Africa's first since 2022, when five cases were reported, none fatal.
The WHO stated, 'Discussions are underway regarding options for vaccine procurement.'
Currently, two mpox vaccines are being deployed in other African countries.

CSL Seqirus today announced that it has commenced shipping its differentiated portfolio of influenza vaccines. This year, the company's influenza vaccines are being produced as trivalent influenza vaccine formulations, in compliance with the U.S. Food and Drug Administration's directive in 2024 to remove the B/Yamagata strain.
For the 2024-2025 influenza vaccine portfolio, CSL Seqirus is the leading manufacturer offering a differentiated influenza vaccine option approved for use in people six months and older.
For example, FLUCELVAX® is the first and only cell-based influenza vaccine indicated for use in people six months and older.
"Influenza continues to pose a significant threat, as evidenced by recent flu seasons," said Dr. Gregg Sylvester, Chief Health Officer, CSL Seqirus, in a press release on July 9, 2024.
"As we begin distributing influenza vaccines to healthcare providers throughout the U.S., it is imperative that we work to maintain high vaccination rates this season to help reduce the burden of influenza-related illnesses and the risk of severe outcomes."
CSL Seqirus is part of CSL, a global leader in the protection of public health and one of the largest influenza vaccine providers in the world.

A virus-like particle (VLP) based vaccine candidate in development to prevent moderate-to-severe acute gastroenteritis (AGE) in infants caused by norovirus infection is being discontinued.
This is unfortunate news since the health burden of norovirus falls disproportionately on young children and older adults.
HilleVax, Inc. today announced topline data results from NEST-IN1, a Phase 2b, randomized, double-blind, placebo-controlled clinical trial to evaluate the efficacy, safety, and immunogenicity of the HIL-214 vaccine candidate in infants of approximately five months of age at the time of initial vaccination at sites in the United States and Latin America.
In the NEST-IN1 study, there were 51 primary endpoint events, with 25 in the vaccine arm (n=1,425) and 26 in the placebo arm (n=1,399), resulting in a vaccine efficacy of 5% (95% confidence interval; -64%, 45%).
The study did not meet its primary efficacy endpoint against moderate or severe AGE events due to GI.1 or GII.4 norovirus genotypes. And no clinical benefit was observed across secondary endpoints. The company plans to discontinue further development of HIL-214 in infants.
HIL-214 did exhibit a safety and immunogenicity profile consistent with what was observed in the prespecified analysis of the first 200 subjects in NEST-IN1 and previously reported studies.
“We are disappointed that the NEST-IN1 study did not meet its primary efficacy endpoint,” said Rob Hershberg, MD, PhD, Chairman and Chief Executive Officer of HilleVax, in a press release on July 8, 2024.
“While HIL-214 previously showed clinical benefit in adults, NEST-IN1 was the first efficacy study conducted in infants for a norovirus vaccine candidate. We believe the efficacy in the infant setting may have been impacted by the appearance of multiple emerging GII.4 strains in this trial.”
The company stated it is exploring the potential for continued development of HIL-214 and HIL-216 in adults, HilleVax’s Phase 1 ready vaccine candidate.
Currently, there are no U.S. FDA-approved norovirus vaccines.
Globally, norovirus is estimated to result in approximately 700 million cases of AGE and 200,000 deaths per year, resulting in over $4 billion in direct health system costs and $60 billion in societal costs per year.
The U.S. CDC publishes information about gastrointestinal illness outbreaks on cruise ships in the Vessel Sanitation Program during 2024, including details and actions taken in response.

The dengue outbreak in the Regions of the Americas continues to expand in 2024, now surpassing 10.2 million cases.
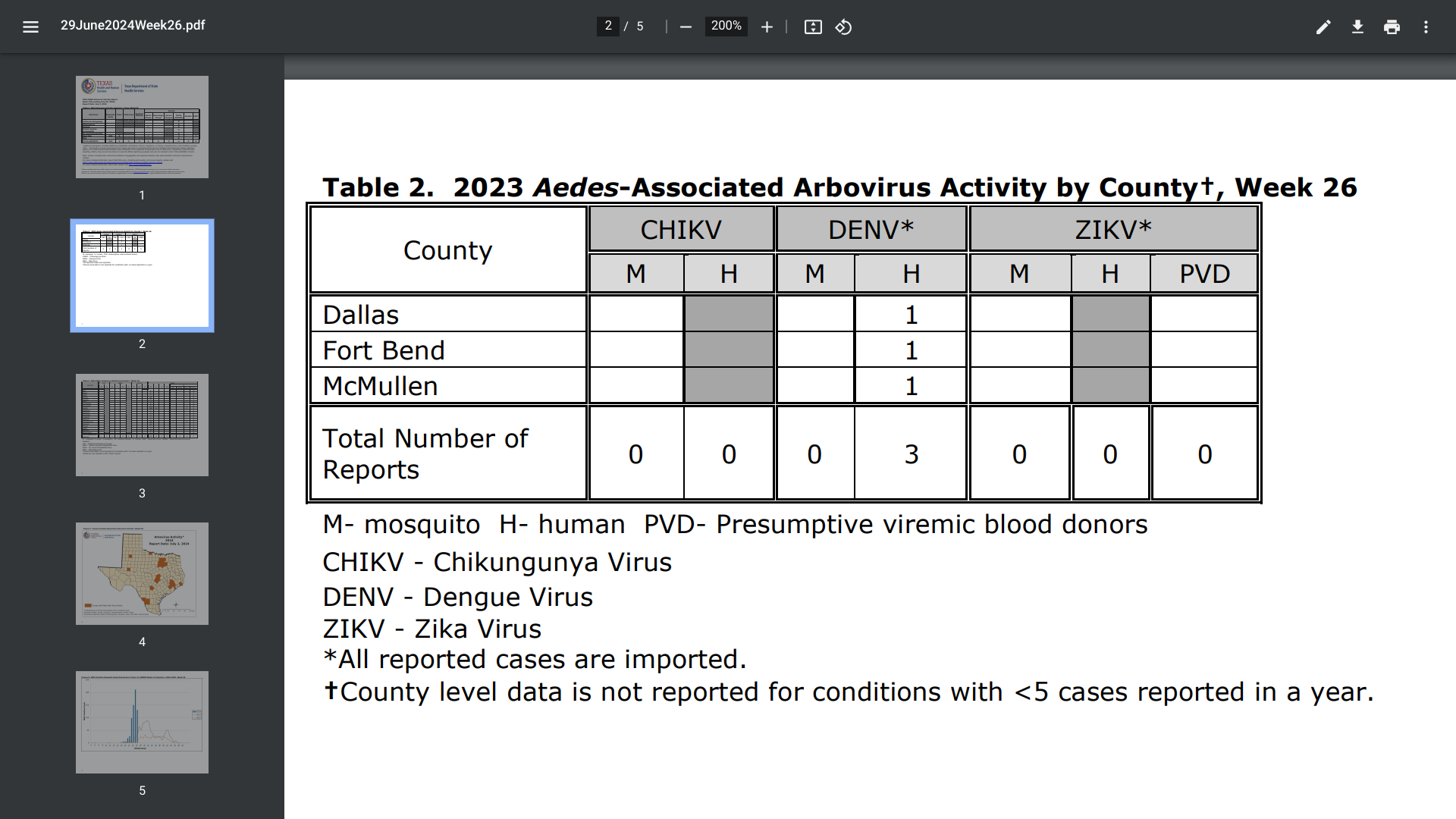
This outbreak has recently reached the state of Texas. Located in Austin, the Texas Department of State Health Services (DSHS) says mosquitoes that transmit dengue fever are found in Texas.
As of July 2, 2024, three dengue cases were reported in Dallas, Fort Bend (Houston), and McMullen counties. The DSHS did not disclose if these were travel-related or local cases.
In 2023, 67 dengue cases were reported in Texas.
To the south of Texas, Mexico continues to report record dengue cases this year.
As of the end of June 2024, the PAHO reported that Mexico had 99,660 dengue cases in 2024. In 2023, the PAHO disclosed that Mexico had reported over 277,000 dengue cases.
The AMA's Vice President of Science, Medicine, and Public Health, Andrea Garcia, JD, MPH, commented in an AMA podcast on July 3, 2024...."So most cases aren't serious, but there can be severe cases that lead to internal bleeding, organ failure, or even death."
"Infections usually begin after an incubation period of about five to seven days, starts with a fever, and then it's accompanied by other symptoms, including nausea, vomiting, rash, muscle aches, joint and bone pain, pain behind the eyes, headache, or low white blood cell counts."
"Unlike other diseases, where fever reduction is a sign that someone's getting better, the critical phase of dengue begins at this time and typically lasts 24 to 48 hours."
"Several warning signs indicate progression to severe disease. Those include abdominal pain or tenderness, persistent vomiting, bleeding from the nose or gums, and lethargy or restlessness."
"Severe disease develops in about one out of 20 people with symptomatic dengue. And infants, pregnant people, adults over 65 years of age, and people with certain medical conditions are at an increased risk."
As of July 8, 2024, approved dengue vaccines are generally unavailable in the United States.
Dengue poses a year-round risk in many parts of the world, with outbreaks occurring frequently. As of July 2024, the United States has reported travel-related and locally acquired cases of dengue fever. This indicates the U.S. is quietly joining the over 100 countries currently facing outbreaks.
As of July 5, 2024, the U.S. Centers for Disease Control and Prevention (CDC) has reported 2,391 dengue cases in 45 jurisdictions.
The unfortunate leader reporting mosquito-transmitted disease is the state of Florida.
Florida Health's latest weekly vector-borne illness report confirmed that ten locally acquired dengue cases were reported from four counties (Miami-Dade (6), Monroe (2), Pasco, and Tampa) in 2024.
In 2023, 186 humans were reported from five Florida counties to have locally contracted dengue.
Additionally, 244 travel-associated dengue cases were reported, mainly by visitors from Brazil and Cuba. In 2023, Florida Health reported 609 travel-associated dengue cases.
While most disease outbreaks of this nature have a variety of preventive vaccines and treatments available, dengue is an anomaly.
The CDC's Health Advisory, dated June 25, 2024, stated, 'No antiviral medications are approved to treat dengue. Treatment is supportive and requires careful volume management.'
"Maintain a high suspicion for dengue among patients with fever and recent travel (within 14 days before illness onset) to areas with frequent or continuous dengue transmission.'
'Healthcare providers should consider locally acquired dengue among patients with signs and symptoms highly compatible with dengue (e.g., fever, thrombocytopenia, leukopenia, aches, pains, rash) in areas with competent mosquito vectors.'
Also, in late June 2024, the CDC informed its vaccine committee that U.S. residents do not currently have access to a previously approved dengue vaccine.
Furthermore, the CDC did not clarify if the second-generation Qdenga® vaccine would be authorized in 2024.

GSK plc recently announced a restructuring of its collaboration agreement with CureVac N.V. Under the new agreement, GSK will focus on the development of mRNA vaccines for influenza and COVID-19 while withdrawing from other infectious disease projects.
As part of the revised contract reported on July 3, 2024, GSK will pay CureVac €400 million (approx. $430 million) upfront. Additionally, GSK has committed to providing up to $1.13 billion in development, regulatory, and sales milestone payments and offering tiered royalties.
Tony Wood, GSK's chief scientific officer, said in a press release, “We are excited about our flu/COVID-19 programs and the opportunity to develop best-in-class mRNA vaccines to change the standard of care. With this new agreement, we will apply GSK’s capabilities, partnerships, and intellectual property to CureVac’s technology to deliver these promising vaccines at a pace.”
This new deal will replace all previous financial terms from the original agreement. In exchange for these payments, GSK will secure full global rights to develop and commercialize CureVac’s investigational mRNA vaccines for influenza and COVID-19, including combination formulations.
Currently, the partners have seasonal flu and COVID-19 shots in Phase II development and an avian flu candidate in Phase I. Both companies believe that these candidates have best-in-class potential.
Completion of the new agreement remains subject to certain antitrust and regulatory approvals and customary closing conditions. The original collaboration between CureVac and GSK was initiated in July 2020.
CureVac is a multinational biotech company founded in 2000 to advance the field of messenger RNA (mRNA) technology for application in human medicine.