Search API
Vaxcyte, Inc. today announced financial results for the second quarter ended June 30, 2024, and provided a business update.
“We continue to make significant strides toward building the potentially best-in-class pneumococcal conjugate vaccine (PCV) franchise and expect to announce the VAX-31 adult Phase 1/2 study topline safety, tolerability and immunogenicity data in September,” said Grant Pickering, Chief Executive Officer and Co-founder of Vaxcyte, in a press release on August 6, 2024.
“Our clinical program assessing VAX-31, the broadest-spectrum PCV in the clinic today, will provide significant insights into the full potential of this vaccine candidate across the adult population."
"Following the VAX-31 adult data readout, we plan to advance either VAX-24 or VAX-31 into Phase 3 clinical development in adults.”
Mr. Pickering continued, “Additionally, we look forward to delivering the topline data from the primary immunization series of the VAX-24 infant Phase 2 study by the end of the first quarter of 2025, followed by topline data from the booster dose by the end of 2025."
"We believe VAX-24 has a potential best-in-class profile for this vital population and is designed to cover more serotypes than any infant pneumococcal vaccine on-market today.”
The Company also confirmed cash, cash equivalents, and investments were $1,851.9 million as of June 30, 2024, compared to $1,242.9 million as of December 31, 2023.

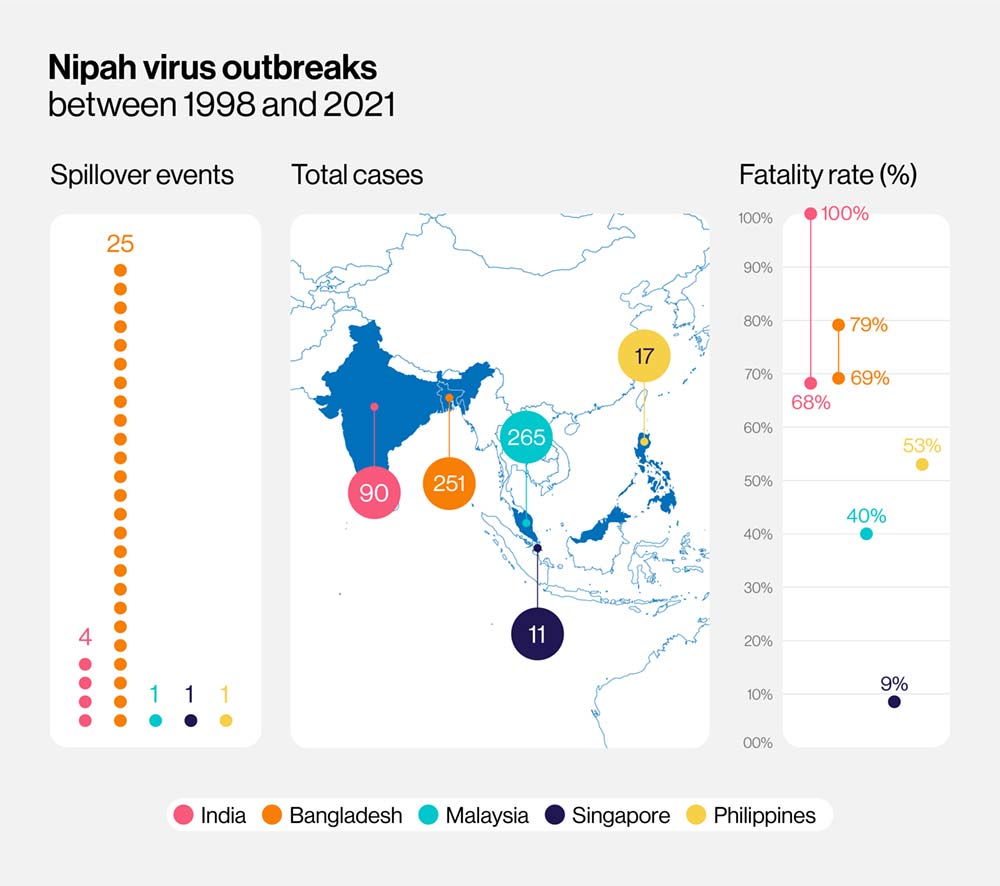
Nipah virus infection is an emerging serious zoonotic disease without a preventive vaccine. This virus is transmitted to humans through infected animals (fruit bats) or food contaminated with excretion and secretions from bats.
Vaccine research is essential since the case fatality rate is estimated at 40% to 75%.
Phylex Biosciences announced today its new mRNA nanoparticle vaccine against the Nipah virus achieved positive results in an immunogenicity study conducted in collaboration with scientists from the U.S. Centers for Disease Control and Prevention (CDC).
The vaccine elicited a robust neutralizing antibody response, with neutralizing titers markedly higher than with several other Nipah vaccine designs and efficient neutralizing even with a single dose. In virus neutralization assays, neutralization titers of Phylex vaccine-elicited sera against Nipah virus were 3-fold the average titers of 14 individuals in Bangladesh who survived a Nipah virus infection.
The Phylex mRNA vaccine encodes for a nanoparticle displaying 60 copies of the antigen-based upon the head domain of the G protein of the Nipah virus.
On August 5, 2024, the company published a preprint of a research article on the immunogenicity of its Nipah mRNA nanoparticle vaccine.
"We are grateful to our co-authors at the CDC for their contribution in assessing our vaccine against this difficult pathogen," said Pascal Brandys, co-founder and CEO of Phylex Biosciences, in a press release.
"The results confirm the strong advantage of our mRNA vaccine encoding for a highly immunogenic nanoparticle, as compared with a variety of other technologies."
"Our vaccine combines the advantages of mRNA for speed of manufacturing and development and a nanoparticle for efficacy after one dose," Brandys continued. "We will aggressively pursue the clinical development of our vaccine candidate to initiate clinical trials with exposed individuals on a compassionate basis and save lives as soon as possible."
The Nipah virus is a pathogen that causes encephalitis and acute respiratory distress in humans. Recent outbreaks have occurred in Bangladesh, India, Malaysia, the Philippines, and Singapore, and the fatality rate is over 50%.
The virus's natural hosts are large fruit bats, which are present across South Asia, including India and Bangladesh.
To assist countries, a Technical Brief was developed In February 2024 as an interim document to guide countries in the readiness planning for a Nipah virus event, especially in countries that have not reported a Nipah virus event.
The CDC has not approved a vaccine or therapeutics against the Nipah virus.
The news agency PTI today reported at least 66 cases of Zika virus infection have been reported in Pune, India, over the last two months. The mosquito-transmitted Zika virus has been reported in India's 16 different states/union territories since 2016.
On August 6, 2024, a senior health official told PTI those infected in Pune, a city of about 7 million, also included 26 pregnant women.
According to the World Health Organization (WHO), there is scientific consensus that Zika infections can cause microcephaly, Guillain-Barré syndrome, and other central nervous system malformations. Additionally, the WHO advises pregnant women to avoid visiting Zika outbreak areas.
As of August 2024, the Region of the Americas has reported over 24,684 ZIka cases in countries such as Brazil, Bolivia, Costa Rica, and Puerto Rico in 2024.
While several Zika vaccine candidates are conducting human clinical trials, no vaccine has been approved.
The African country of Mozambique today reached an important milestone in malaria prevention by introducing the R21/Matrix-M™ vaccine. This innovative vaccine brings the number of African countries offering malaria vaccines to eleven.
This vaccination program is essential since malaria is endemic in Mozambique, with a prevalence of 32% in children.
Through Gavi, the Vaccine Alliance, and co-financing from the Government of Mozambique, the country will vaccinate around 300,000 children through the country’s Expanded Programme on Immunization.
“The malaria vaccine, which is being rolled out initially in Zambezia today, is one of the latest approaches in the fight against the disease,” said Hon Dr Armindo Tiago, Minister of Health, in a press release on August 5, 2024.
“The choice of Zambezia as the launch site is due to the province's high burden of the disease. The vaccine will be administered in four doses to reduce the severe malaria illness and death.”
The World Health Organization (WHO) recommended that the malaria vaccine be administered in a 4-dose schedule, with the first dose covering children aged 6 to 11 months. A more extensive malaria vaccine rollout is expanding access to prevent additional disease.
Previously, the R21/Matrix-M™ malaria vaccine was launched in Côte d'Ivoire.
John Jacobs, Novavax Inc.'s President and CEO, said in a press release on July 15, 2024, ".... marks a breakthrough in the fight to protect vulnerable children against a leading cause of death across the region while reinforcing our mission to create innovative vaccines that improve public health."
As of August 6, 2024, malaria vaccines are not offered in the United States. However, the majority of travel-related malaria cases diagnosed in the U.S. originate from African travelers.
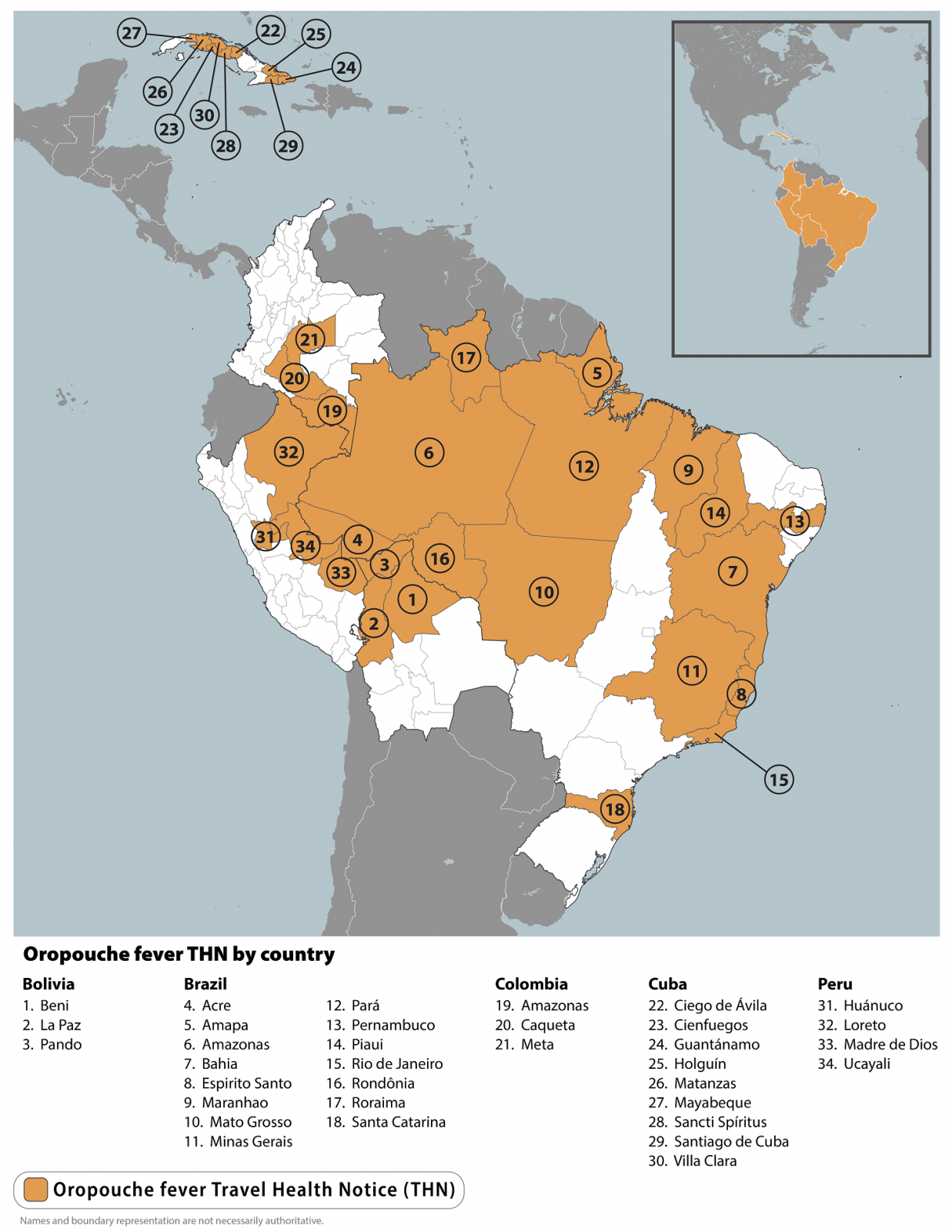
The U.S. Centers for Disease Control and Prevention (CDC) recently issued a Level 1 Travel Health Advisory confirming outbreaks of Oropouche fever in parts of Brazil, Bolivia, Colombia, Peru, and Cuba.
On August 1, 2024, the CDC reported that 34 countries had reported 8,078 Oropouche fever cases this year.
Local transmission has also been documented in ten non-Amazonian states, some without previous cases reported. In Brazil, 7,284 cases were confirmed, mainly in the Amazon region.
Travelers to affected areas should avoid infected midges (small flies) and mosquitoes as they spread this disease, which is often mistaken for dengue. The CDC says travelers should seek medical care if they develop high fever, headache, muscle aches, stiff joints, nausea, vomiting, chills, or sensitivity to light during or after travel.
According to reports, Oropouche fever can be passed between a pregnant woman and an unborn child.
As of July 30, 2024, five potential cases of vertical transmission have been identified in Brazil: four cases of stillbirth and one case of spontaneous abortion.
On July 17, 2024, the Pan American Health Organization (PAHO) said it is not clear if infection with the Oropouche virus caused adverse health outcomes for the fetuses.
The CDC is working with PAHO and other international partners to learn more about the potential risks of Oropouche during pregnancy.
As of August 6, 2024, there are no approved vaccines to protect people from this disease.
ImmunityBio, Inc., today announced the opening of the National Cancer Institute-sponsored Phase 1/2 QUILT 502 clinical trial, which will study ANKTIVA® together with the investigational AdHER2DC vaccine in individuals with HER2-expressing endometrial cancer.
It marks the latest trial involving ANKTIVA, the company’s IL-15 superagonist immune enhancer. The aim of the trial is to evaluate ANKTIVA as an agent to replace the short-term activity of checkpoint inhibitor immunotherapies with long-term effectiveness.
Endometrial cancer is the most common gynecological cancer in the U.S. and affects more than 65,000 women each year, with incidence peaking around 50-60 years of age. The 5-year overall survival rate in patients with metastasis is around 20 percent; treatment options after the second-line treatment are limited.
The AdHER2DC vaccine targets the HER2 protein, which is elevated in 30% of patients with endometrial cancer and in more than 50% of high-risk subtypes. The single agent AdHER2DC demonstrated a safety profile and immunogenicity in a phase 1 clinical trial.
The U.S. FDA recently approved ANKTIVA for BCG-unresponsive non-muscle invasive bladder cancer CIS with or without papillary tumors.
“We are pleased to partner with the NCI on this important cancer control study involving ANKTIVA, which has demonstrated in clinical trials that activation of memory T cells may help deliver long-duration responses well beyond that of checkpoint inhibitors alone,” said Patrick Soon-Shiong, M.D., Executive Chairman and Global Chief Scientific and Medical Officer at ImmunityBio, in a press release on August 6, 2024.
“We are hopeful that the AdHER2DC investigational vaccine plus ANKTIVA will ‘rescue’ the checkpoint inhibitor pembrolizumab and kinase inhibitor lenvatinib and lead to an improved response compared with the current standard of care in this high-risk population.”
Phase 1 of the open-label, two-arm Phase 1/2 study will determine the recommended dose of pembrolizumab, lenvatinib, ANKTIVA, and AdHER2DC in participants with HER2-positive endometrial cancer.
The Phase 2 portion of the study will assess the efficacy of the combination of pembrolizumab, lenvatinib, ANKTIVA, and the AdHER2DC vaccine in qualified participants as determined by the proportion of participants without disease progression at six months. The study will enroll 60 subjects and is expected to be completed in 2026.
ImmunityBio says these studies, along with the recent approval of ANKTIVA for bladder cancer, signal the advent of the era of cytokines as the next generation of immunotherapies.
To combat one of the most lethal forms of pediatric brain cancer, UCLA Health Jonsson Comprehensive Cancer Center researchers are launching a first-of-its-kind clinical trial to evaluate the safety and effectiveness of a cancer vaccine candidate targeting H3 G34-mutant diffuse hemispheric glioma.
This highly aggressive brain tumor is typically found in adolescents and young adults.
A particular mutation of the H3-3A gene primarily characterizes this type of brain tumor. This mutation leads to significant disruptions in RNA processing, with wide-ranging influences on cancer behavior and response to treatment.
The vaccine candidate developed at UCLA targets these tumor genetic mutations. UCLA Health is the only center investigating immunotherapy for this type of glioma.
“Despite aggressive treatments, this type of brain tumor evades current therapies with shocking efficiency,” said Dr. Anthony Wang, director of the Pediatric Brain Tumor Program at UCLA Health and the principal investigator of the trial, in a press release on August 5, 2024.
“These cancers show a host of escape pathways, allowing small populations of cells to survive initial treatment and to adapt. The data from our pre-clinical studies makes us hopeful that an active, targeted cancer vaccine will be able to adapt with the tumor to eliminate cancer cells more effectively.”
The vaccine works by arming a patient’s dendritic cells, the most efficient activator of the body’s immune system, to target products of the altered RNA regulation that defines this cancer type.
Once activated against these targets, the patient’s dendritic cells are injected back into the patient.
Dendritic cell vaccination has already shown promise in treating some other forms of cancer, including glioblastoma, adding years of life for a subset of patients with a disease that often only has a lifespan of months.

Biopharmaceutical New Technologies (BioNTech) SE today announced about €807.8 million in losses in the second quarter of 2024. This negative report compares to $208 million during the same period in 2023. The increased operating loss was impacted by decreased demand for mRNA COVID-19 vaccines.
From a working capital perspective, the Company ended the second quarter of 2024 with €18.5 billion in cash, cash equivalents, and security investments.
Prof. Ugur Sahin, M.D., CEO, and Co-Founder of BioNTech, commented in a press release on August 5, 2024, “In addition, we have started commercializing variant-adapted COVID-19 vaccines for the upcoming season while accelerating our clinical development efforts to realize the full potential of our technologies."
On July 24, 2024, the United Kingdom’s Medicines and Healthcare products Regulatory Agency approved the companies’ Omicron JN.1-adapted vaccine.
Sahin added, "We are making progress towards our goal of becoming a company with marketed medicines for cancer and infectious diseases.”
BioNTech is also working on expanding its infectious diseases portfolio beyond COVID-19 with continued investments in influenza and cancer, with the BNT111 Melanoma mRNA immunotherapy candidate.