Search API
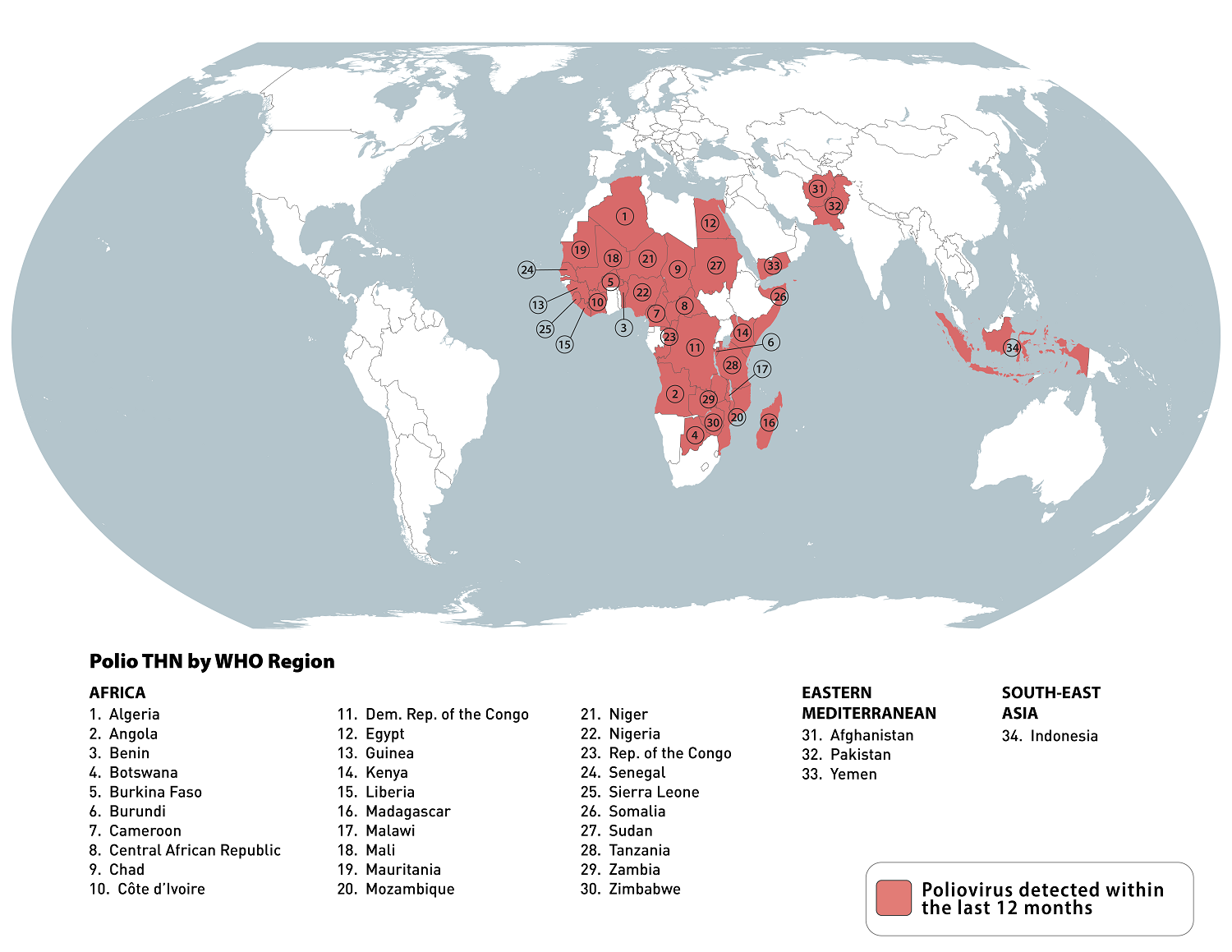
According to the weekly update from the Global Polio Eradication Initiative (GPEI), six countries reported poliovirus cases, including Afghanistan and Pakistan, with additional wild poliovirus type 1 (WPV1) cases.
The GPEI says both countries have already doubled the cases they reported in 2023.
As of August 7, 2024, Afghanistan reported two new WPV1 cases, bringing its total to 11.
Pakistan reported three more cases, increasing its total to 12 for 2024.
Four African countries reported cases involving circulating vaccine-derived poliovirus type 2 (cVDPV2).
The Democratic Republic of the Congo reported two cVDPV2 cases. Ethiopia reported one case, boosting its total to 12. Nigeria reported one case, elevating its total to 38, and South Sudan reported its 7th cVDPV2 case for 2024.
The U.S. CDC says most people with polio do not feel sick. Some people have only minor symptoms, such as fever, tiredness, nausea, headache, nasal congestion, sore throat, cough, stiffness in the neck and back, and pain in the arms and legs.
In rare cases, polio infection causes permanent loss of muscle function.
Polio can be fatal if the breathing muscles are paralyzed or if the brain is infected.
Furthermore, the CDC recommends that adults who previously completed the full, routine polio vaccine series may receive a single, lifetime booster dose of polio vaccine before traveling to any destination reporting polio cases. Polio vaccines are generally available at health clinics and pharmacies in the U.S.
HilleVax, Inc., a company focused on developing and commercializing novel vaccines, announced financial results for the June 30, 2024 quarter and highlighted recent progress.
As of June 30, 2024, and December 31, 2023, the company had cash, cash equivalents, and marketable securities totaling $245 million and $303.5 million, respectively.
On August 8, 2024, the company confirmed it is exploring the potential for continued development of its HIL-214 and HIL-216 norovirus vaccine candidates in adults.
However, the company has discontinued further development of HIL-214 in infants.
This is unfortunate news since no U.S. FDA-approved norovirus vaccines are available to meet disease prevention needs.
The U.S. CDC recently reported norovirus is the leading cause of vomiting and diarrhea from acute gastroenteritis among people of all ages and causes 58% of foodborne illnesses acquired in the United States.
Each year, there are about 2,500 reported norovirus outbreaks in the U.S., including on cruise ships.

According to the World Health Organization (WHO), the global Dengue fever outbreak continues to expand in August 2024.
Dengue outbreaks are being reported by 90 countries in 2024, with most of these cases reported in the Region of the Americas.
As of August 8, 2024, 43 countries and territories in the Region have reported over 11.1 million Dengue cases and about 6,135 related deaths this year.
The updated data is over 120% greater than recorded throughout 2023.
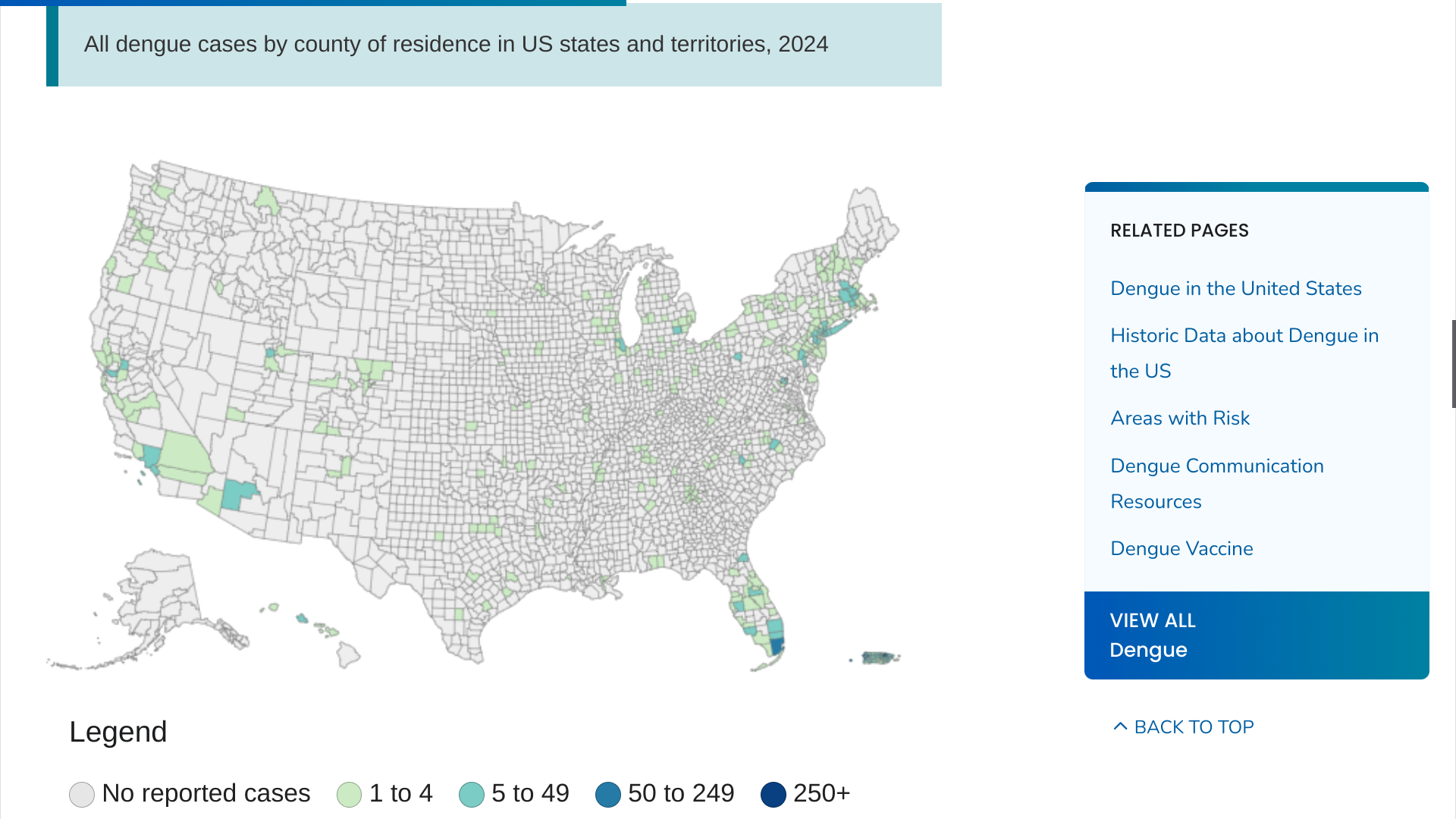
In the United States, the U.S. CDC reported on August 7, 2024, that 52 jurisdictions, led by Florida, New York/New Jersey, and Puerto Rico, had confirmed 3,290 dengue cases.
In 2023, only 2,343 Dengue cases were reported to the CDC.
The CDC says Dengue is endemic in the U.S. territories of Puerto Rico, American Samoa, the U.S. Virgin Islands, the Federated States of Micronesia, the Republic of Marshall Islands, and the Republic of Palau.
Currently, the CDC says the best way to prevent this mosquito-transmitted disease is to wear protective clothing, as no Dengue vaccine is available in the U.S.
However, in 2024, Takeda's QDENGA® (TAK-003) two-dose vaccine is available in over 20 countries. The WHO added QDENGA to its List of Prequalified Vaccines effective May 9, 2024.
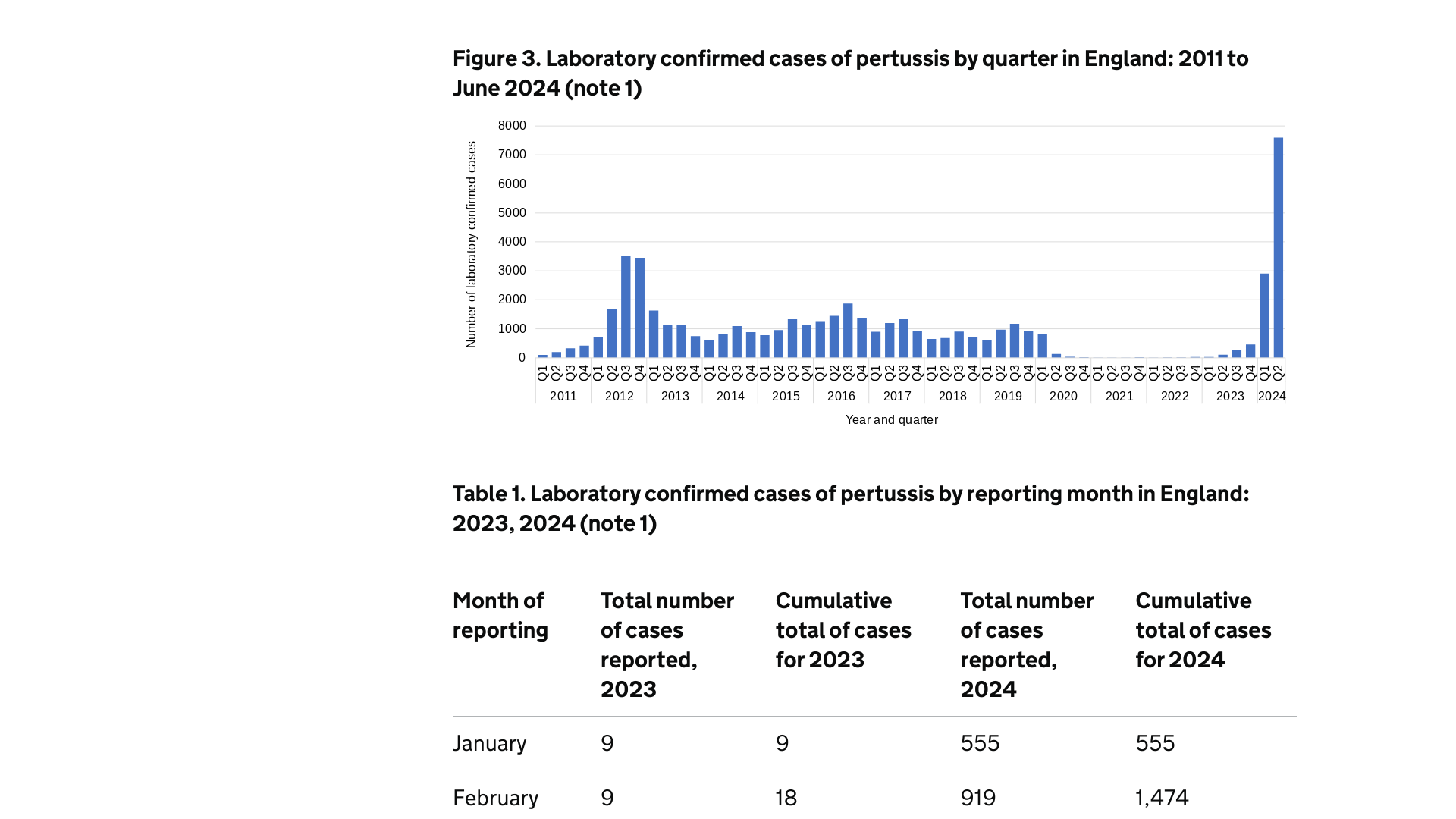
New whooping cough data published today by the U.K. Health Security Agency (UKHSA) shows that laboratory-confirmed cases have exceeded 10,400.
The latest data for England shows cases of whooping cough peaked in May 2024 but continue at high levels, with 2,427 cases reported in June.
On August 8, 2024, the UKHSA confirmed one additional infant death in June, bringing the total to 10 since the current outbreak, which began in November 2023.
Evidence from England shows that vaccination at the right time in pregnancy is highly effective, offering 92% protection against infant death.
The latest uptake data for the vaccination offered to pregnant women to protect newborn infants against whooping cough continues to decline - with coverage in March 2024 at 58.9% compared to the peak coverage (72.6%) in March 2017.
Dr. Mary Ramsay, Director of Immunisation at the UKHSA, said in a press release, "Vaccination is the best defense against whooping cough, and pregnant women and young infants must receive their vaccines at the right time. Pregnant women are offered a whooping cough vaccine in every pregnancy, ideally between 20 and 32 weeks."
"This (vaccination) passes protection to their baby in the womb so that they are protected from birth in the first months of their life when they are most vulnerable and before they can receive their vaccines."
Whooping cough, also known as pertussis, is a bacterial infection that affects the lungs. The first signs of infection are similar to a cold, such as a runny nose and sore throat, but after about a week, the infection can develop into coughing bouts that last for a few minutes and are typically worse at night, says the UKHSA.
In the United States, whooping cough vaccines are generally available at health clinics and pharmacies.
As of August 2024, the U.S. CDC has not issued a Travel Health Advisory regarding the U.K.'s whooping cough outbreak.
Novavax, Inc. today announced its financial results and operational highlights for the second quarter ended June 30, 2024. The Company confirmed it achieved total revenue of $415 million in the second quarter of 2024 and ended the period with $1.1 billion in Cash.
John C. Jacobs, President and Chief Executive Officer of Novavax, commented in a press release on August 8, 2024, "We intend to drive future value for the business through not only the Sanofi partnership but also through our late-stage combination and influenza assets."
"We plan to unveil a new and expanded clinical pipeline by the end of this year and leverage the pipeline and our proven technology to drive additional partnerships and deals and ultimately drive significant, long-term value for our shareholders."
Additionally, Novavax has taken steps to enable a successful operationalization of the collaboration and license agreement with Sanofi Pasteur Inc.
Effective January 1, 2025, Sanofi will assume primary commercial responsibility for Novavax's updated 2024-2025 formula COVID-19 vaccine (NVX-CoV2705) in the U.S., Europe, and select major markets not currently subject to Novavax Advance Purchase Agreements or existing partnership agreements.
Furthermore, the Company expects to deliver its updated 2024-2025 formula protein-based COVID-19 vaccine to the market by the start of the season, and it has advanced retail pharmacy contract negotiations to enhance access for the 2024-2025 vaccination season.
The COVID-19 vaccine was created using Novavax's nanoparticle technology, Matrix-M™, an adjuvant that enhances immune responses and stimulates high levels of neutralizing antibodies.
In the U.S. market, Novavax submitted an Emergency Use Authorization (EAU) amendment to the U.S. Food and Drug Administration (FDA); doses will be ready to ship upon receipt of the EUA. The FDA accepted the Biologics License Application for Novavax's COVID-19 vaccine with a Prescription Drug User Fee Act date of April 2025.
However, the U.S. FDA has not approved the trade name Nuvaxovid™.

Bavarian Nordic A/S today announced that it had received a new order valued at USD 156.8 million from the U.S. Biomedical Advanced Research and Development Authority (BARDA) to manufacture additional bulk product for JYNNEOS® (MVA-BN®, IMVAMUNE®, IMVANEX®), the company’s smallpox/mpox vaccine.
The new BARDA contract will help replenish the inventory of bulk vaccines required for future manufacturing and supply of freeze-dried vaccines.
The bulk product, representing $139.7 million of the contract value, will be manufactured and invoiced in 2024 and will partly replenish the inventory used to manufacture vaccines in response to the global mpox outbreak that began in May 2022.
Replenishment of the bulk inventory is necessary to fulfill the company’s existing contract to supply a next-generation, freeze-dried version of the vaccine for U.S. smallpox preparedness.
Since 2003, Bavarian Nordic has worked with the U.S. government on the development, manufacturing, and supply of a non-replicating smallpox vaccine.
The vaccine was approved by the U.S. FDA in 2019 under the trade name JYNNEOS®, which is indicated for preventing both smallpox and mpox infection.
In real-world studies, JYNNEOS effectiveness against mpox disease adjusted vaccine effectiveness estimates ranged from 35% (95% CI, -2-59) to 89% (95% CI, 76-95) after one dose and from 66% (95% CI, 47-78) to 90% (95% CI, 86-92) after two doses.
Before FDA approval, Bavarian Nordic had supplied nearly 30 million doses of the liquid-frozen version to the U.S., with the vast majority being delivered for emergency use - and now expired.
“Our smallpox/mpox vaccine represents a key component in the U.S. biological preparedness, as demonstrated during the 2022 mpox outbreak," said Paul Chaplin, President & CEO of Bavarian Nordic, in a press release on August 8, 2024.
"JYNNEOS was also the first smallpox vaccine successfully developed under Project BioShield, a program created by the U.S. Congress in 2004 to accelerate the research, development, procurement, and availability of medical countermeasures against biological, chemical, radiological, and nuclear agents through public-private partnerships."
"We applaud the U.S. government’s steadfast commitment to maintaining a robust preparedness and are proud to continue providing vaccines to protect its citizens against current and future public health threats,” added Chaplin.
The new BARDA contract also includes approximately $17 million for additional services in 2025-2027, including storage of vaccine doses in the U.S.
BARDA has supported the development of a freeze-dried version of the vaccine with a longer shelf life to replace the stockpile and awarded the company a ten-year contract for the supply of freeze-dried vaccines in 2017.
On March 14, 2024, the U.S. CDC's Agam Rao, MD CAPT, U.S. Public Health Service, stated, 'JYNNEOS vaccination is expected to be effective regardless of mpox clade.'
As of August 2024, the JYNNEOS vaccine is commercially available in the United States. Healthcare providers in the U.S. administer JYNNEOS for no charge, regardless of any administration fee. The CDC does not endorse booster doses (3rd).

According to Avian Flu Diary's report on August 6, 2024, Finland is offering bird flu vaccination to qualifying adults over 18 who, due to work or other circumstances, have an increased risk of contracting it.
Finland has received 20,000 doses of the H5N8 bird flu vaccine, enough to vaccinate 10,000 people with two doses.
The batch of vaccines received in Finland expires at the end of September 2024, so those at risk of infection should take the first vaccine dose in August.
So far, there are no human bird flu infections in Finland. Taking the bird flu vaccine is voluntary in Finland.
On July 15, 2024, a U.S. government spokesperson confirmed in an emailed statement, 'Avian influenza vaccination has not been recommended for any segment of the population, and the U.S. government continues to monitor the situation.'
As of August 7, 2024, the U.S. vaccine stockpile has access to various avian influenza vaccines, such as Audenz.