Search API
Emergent BioSolutions today announced it has pledged to donate 50,000 doses of its ACAM2000® (Smallpox (Vaccinia) Vaccine, Live) through a humanitarian relief organization to the Democratic Republic of the Congo (DRC) and the other impacted countries of Burundi, Kenya, Rwanda, and Uganda.
In October 2023, Emergent filed a supplemental Biologics License Application to the U.S. FDA seeking an expanded indication for the ACAM2000 vaccine to include immunization against the mpox virus. The FDA target for review completion in the third quarter of 2024.
These efforts are in response to the WHO’s recent statement declaring that the upsurge of mpox clade 1 in African countries constitutes a public health emergency of international concern under the International Health Regulations.
“Africa CDC estimated they will need 10 million doses to control the epidemic in the continent,” said Dr. Raina McIntyre, Professor of Global Biosecurity, NHMRC L3 Research Fellow, Head, Biosecurity Program, Kirby Institute, University of New South Wales Sydney, in a press release on August 19, 2024.
“It is unlikely there will be enough supply of 3rd generation vaccines (JYNNEOS®, MVA-BN®) to control the epidemic in Africa, given demand in other countries.”
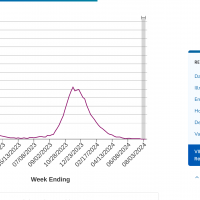
A recent study found that the Fluad® MF59-adjuvanted influenza vaccine (aTIV) was more effective than the high-dose flu vaccine (HD-TIV) at preventing severe respiratory complications in older adults with risk factors.
Published in Open Forum Infectious Diseases on August 16, 2024, the study included 1,115,725 aTIV and 2,561,718 HD-TIV recipients. For the primary outcome, the analysis found comparable effectiveness between aTIV and HD-TIV (rVE [95% CI]: 5.2% [-5.9–15.1]) among those with 0 risk factors, whereas aTIV was more effective than HD-TIV among patients with ≥1, 1–2, or ≥3 risk factors (12.5% (10.0–15.0), 18.4% (13.7–22.9), and 10.4% (7.4–13.3), respectively).
The same trends were observed for the secondary outcomes.
Previous studies have found the two vaccines to be similar in effectiveness in older adults.
The Fluad vaccine has an extensive clinical legacy and has been licensed in 30 countries since its first approval in 1997. Fluad is available at most pharmacies in the U.S. for the 2024-2025 flu season.

Throughout 2024, Cuba has been grappling with an outbreak of Oropouche Fever. And now, its western neighbor, the United States, has started to report cases related to travelers from Cuba.
The Florida Department of Health (FDH) has recently reported 11 Oropouche Fever cases.
As of August 10, 2024, these Florida cases had their onset in 2024 and were found in individuals who had traveled to Cuba two weeks before showing symptoms.
The Oropouche reported cases were found in the following Florida counties: Hillsborough (4), Lee (2), Miami-Dade (1), Orange (2), and Polk (2).
Throughout 2024, more than 8,000 Oropouche cases, including two deaths and five cases of vertical transmission, were reported by the U.S. CDC.
According to the CDC, approximately 60% of people infected with the Oropouche virus become symptomatic. The incubation period is typically 3–10 days. Although people exposed to biting midges or mosquitoes infected with the virus are most at risk for developing the disease, the risk factors for more severe Oropouche virus are not well-defined.
The initial clinical presentation is similar to diseases caused by dengue, Zika, and chikungunya viruses.
In the U.S., healthcare providers should contact local health departments to facilitate diagnostic testing.
As of August 19, 2024, no approved Oropouche vaccines are available.
In addition to Oropouche cases, FDH reported 18 locally acquired dengue fever virus cases and numerous travel-related dengue cases as of week #32.
The World Health Organization (WHO) today announced temporary (one-year) recommendations for States Parties experiencing the upsurge of monkeypox virus (MPXV) clade 1 detections, including, but not limited to, the Democratic Republic of the Congo (DRC), Burundi, Kenya, Rwanda, and Uganda.
The upsurge of mpox cases in the DRC in 2024 and its neighboring countries is driven by outbreaks associated with two sub-clades of clade I MPXV: clade Ia and clade Ib.
These WHO recommendations include establishing or strengthening cross-border collaboration arrangements for surveillance and management of suspect mpox cases and providing information to travelers and conveyance operators without resorting to general travel and trade restrictions unnecessarily impacting local, regional, or national economies.
As of August 19, 2024, the WHO Committee considered the event “extraordinary” because of the increase in mpox clade I disease occurrence in the DRC and the emergence of the new MPXV clade Ib.
Clade I mpox was classically described in studies conducted by WHO in the 1980s to have a mortality rate of approximately 10%, with most deaths occurring in children.
MPXV clade Ia is endemic in the DRC. The disease primarily affects children. Data available for 2024 show an aggregated case fatality rate of 3.6%, and the spread is likely sustained through multiple modes of transmission, including person-to-person transmission following zoonotic introduction in a community.
MPXV clade Ib is a new strain of MPXV that emerged in the DRC. It is transmitted between people, presumed via sexual contact, which has been spreading in the eastern part of the country.
Although first characterized in 2024, estimates suggest it emerged around September 2023.
The outbreak associated with clade Ib in the DRC primarily affects adults and is spreading rapidly, sustained largely, but not exclusively, through transmission linked to sexual contact and amplified in networks associated with commercial sex and sex workers.
Furthermore, these African countries are to initiate plans to advance mpox vaccination activities targeting people at high risk of infection. As of August 19, 2024, various reports indicate that (10 million) mpox vaccines are being produced to meet potential outbreak demand.
In early August 2024, the U.S. CDC issued a Level 2 - Practice Enhanced Precautions, Travel Health Advice, recommending various mpox protection tactics, including (JYNNEOS) vaccination.
These new WHO recommendations are intended to be implemented by those States Parties in addition to the current standing recommendations for mpox, which will be extended until August 20, 2025.

With the World Health Organization announcing new alerts regarding the mpox virus clade 1 outbreak in African countries and Europe confirming travel-related cases, many governments were concerned about vaccine supply.
To alleviate those concerns, Bavarian Nordic (BN) A/S recently announced an update on plans to produce additional JYNNEOS® (MVA-BN®, IMVAMUNE®) vaccine supplies to tackle the current mpox outbreak.
On August 17, 2024, BN wrote, It would appear that mpox will remain a constant threat to public health, and the company is working closely with the Africa CDC to expand further the manufacturing capacity to produce the mpox vaccine in Africa through transfer of technology to selected African manufacturers.
BN has informed the Africa CDC that, in addition to current orders, it has the capacity to manufacture 10 million doses by the end of 2025 and could already supply up to 2 million doses in 2024.
"We are prepared to work with the Africa CDC and the international community to play our role in protecting and saving lives around the World and to contain the latest outbreak,” said Paul Chaplin, President & CEO of Bavarian Nordic, in a press release.
Since the mpox clade 2 outbreak began in May 2022, BN has supplied more than 15 million doses of the mpox vaccine to more than 76 countries.
While several vaccine efficacy studies have reported JYNNEOS's efficacy against clode 2 between 20% and 80%, studies on clade 1 efficacy are pending.
In the United States, the JYNNEOS vaccine remains available at specific clinics and pharmacies.

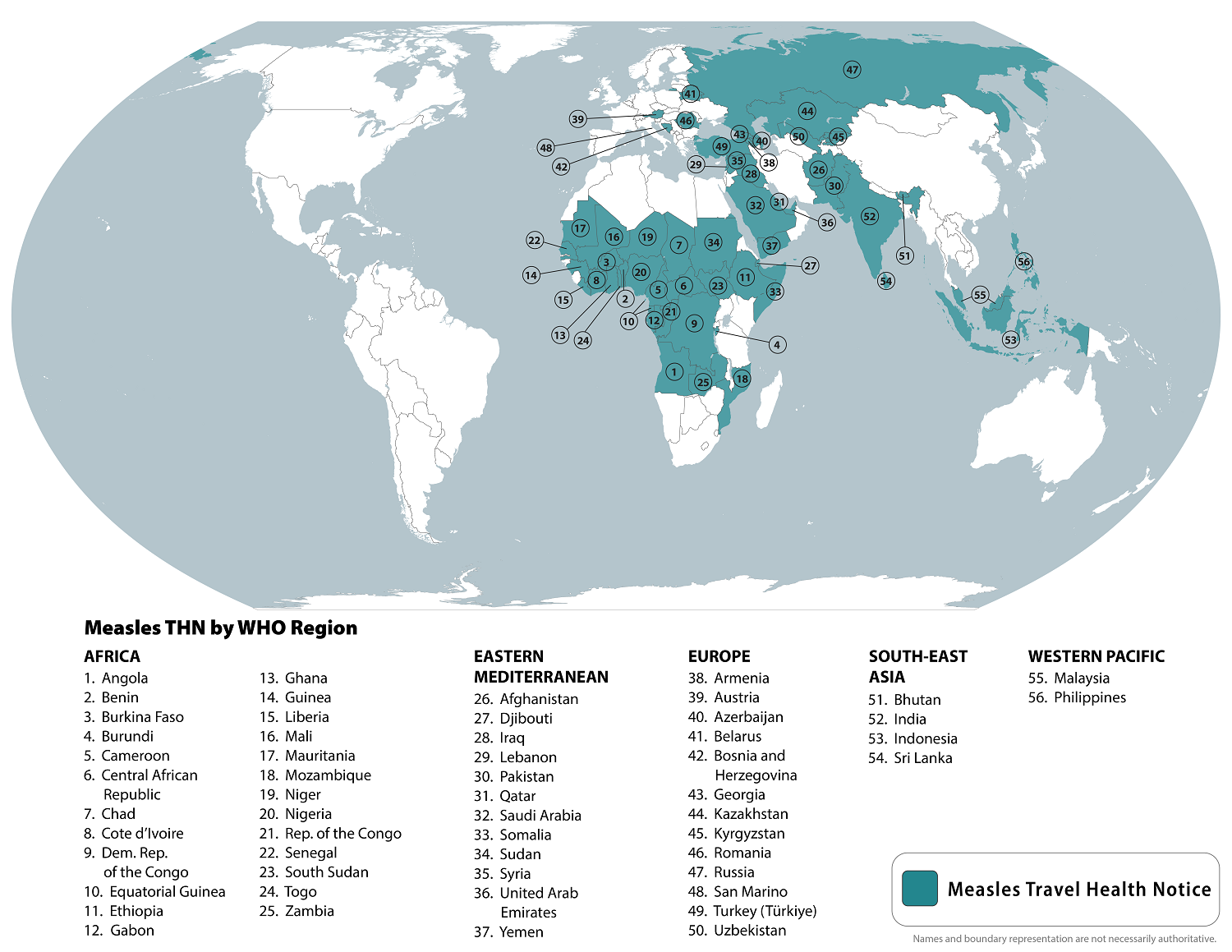
The World Health Organization (WHO) recently reported that measles outbreaks continue in various countries in 2024, such as India.
To alert international travelers, the U.S. Centers for Disease Control and Prevention (CDC) republished a global Watch-Level 1, Practice Usual Precautions, Travel Health Notice on August 14, 2024, identifying measles outbreaks in 56 countries.
Within the United States, the CDC has reported 219 measles cases in 27 jurisdictions this year, led by the states of Illinois and Oregon. Most of these cases are related to unvaccinated travelers.
The CDC writes that 'all international travelers should be fully vaccinated against measles with two doses of the measles-mumps-rubella (MMR) vaccine, including an early dose for infants 6–11 months.
However, the CDC does not recommend a third dose of MMR vaccination during or when visiting measles outbreaks.
In the U.S., MMR vaccines are available at most clinics and community pharmacies.
The World Health Organization (WHO) recently announced that mass mpox vaccination is not recommended against this sexually transmitted disease.
On August 17, 2024, the WHO confirmed international travelers who may be at risk based on an individual assessment with their healthcare provider may wish to consider vaccination before visiting countries reporting mpox outbreaks, such as in Africa.
Recently, the WHO Director-General announced that he had triggered the process for Emergency Use Listing of mpox vaccines. The WHO currently recommends two new vaccines against mpox disease and continues listing an older smallpox vaccine as an option.
The United States Food and Drug Administration (FDA) Approved Bavarian Nordic's JYNNEOS® (MVA-BN, IMVANEX®, IMVAMUNE®) Smallpox and Mpox Vaccine on September 24, 2019. The U.S. began offering the JYNNEOS vaccine to healthcare staff in Boston on May 24, 2022, in response to the Clade 2 mpox global outbreak.
JYNNEOS remains available in the U.S. at certain clinics and pharmacies.
Additionally, Japan's K.M. Biologics' LC16 "KMB" freeze-dried smallpox vaccine has been approved by the WHO, Japan's Ministry of Health, Labor, and Welfare, and other countries.
The older ACAM2000® live vaccinia virus vaccine is authorized to prevent mpox and smallpox infections in various countries. However, the safety profile of ACAM2000 vaccination includes risks for myocarditis and pericarditis.
The WHO writes, 'Results from vaccine effectiveness studies indicate that a good level of protection is provided against mpox (Clade 2) following vaccination. Further studies on the use of vaccines for mpox (Clade 1) will provide additional information on the effectiveness of these vaccines in different settings.'
The Centers for Disease Control and Prevention (CDC) today issued a Health Alert Network Health Advisory (CDCHAN-00515) to notify clinicians and public health authorities of an increase in Oropouche virus disease cases confirmed in the Americas region.
The CDC issued this alert because the initial clinical presentation of the Oropouche virus may confuse providers as the symptoms are similar to those of dengue, Zika, and chikungunya.
Between January and August 1, 2024, more than 8,000 cases of Oropouche virus disease were reported, including two deaths and five cases of vertical transmission associated with fetal death or congenital abnormalities.
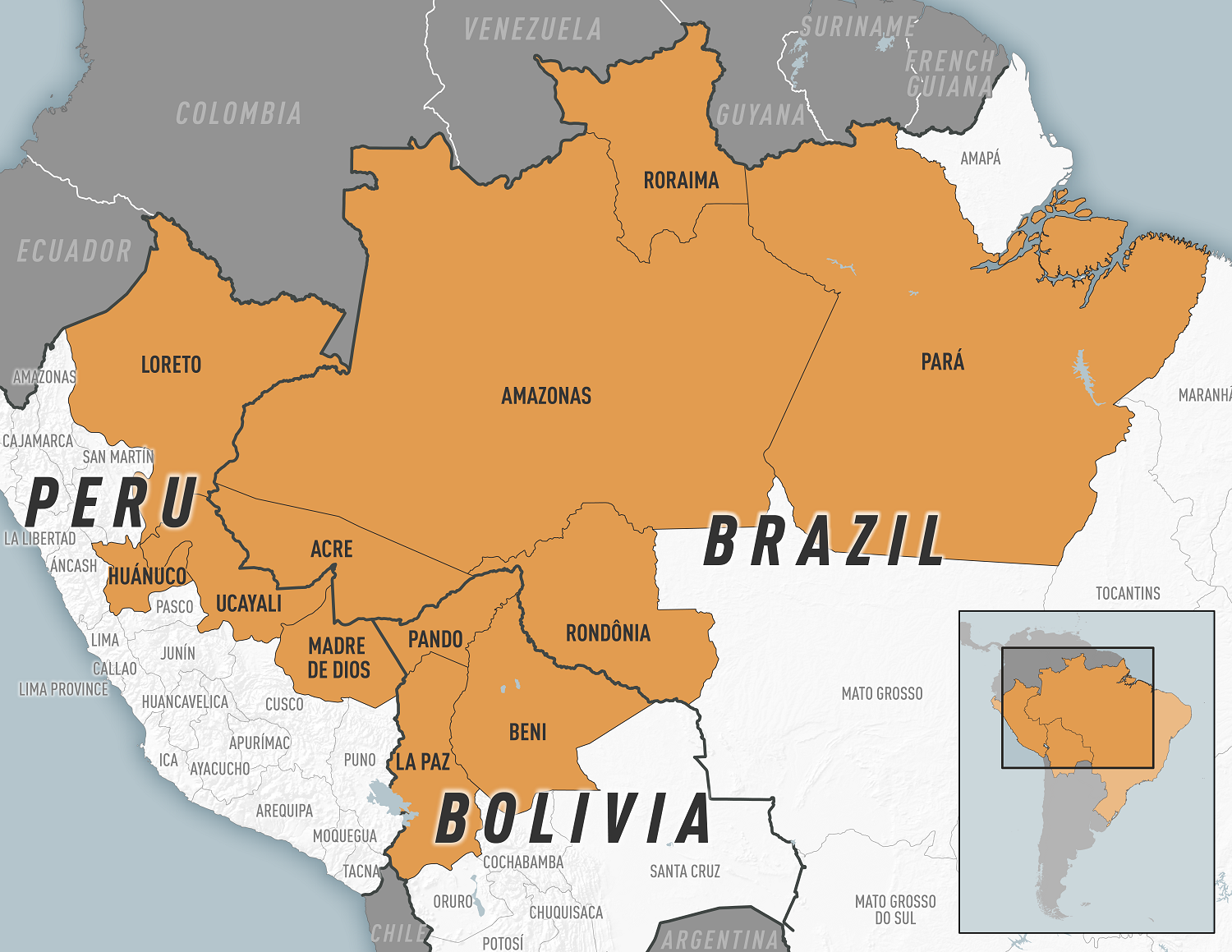
These Oropouche cases originate from endemic areas in the Amazon basin and regions in South America and the Caribbean.
The virus was first detected in 1955 in Trinidad and Tobago and is endemic in the Amazon basin.
As of August 16, 2024, Brazil, Bolivia, Peru, Colombia, and Cuba were among the countries reporting cases. As testing and surveillance for Oropouche virus disease increase in the Americas, reports of cases from additional countries are expected.
Throughout 2024, travel-associated cases have been identified in travelers returning to the United States and Europe from Cuba and Brazil.
This CDC Health Advisory offers advice on evaluating and testing travelers who have been in impacted areas with signs and symptoms consistent with Oropouche virus infection.
It also raises awareness of the possible risk of vertical transmission (e.g., from gestational parent to fetus during pregnancy) and associated adverse effects on pregnancy.
The CDC issued a Level 2 Travel Health Notice in August 2024, suggesting pregnant women reconsider non-essential travel to areas with Oropouche virus outbreaks, such as Brazil and Cuba.
The new alert also highlights prevention measures to mitigate the additional spread of the virus and potential importation into unaffected areas, including the U.S.
Oropouche virus belongs to the Simbu serogroup of the genus Orthobunyavirus in the Peribunyaviridae family.
According to the CDC, approximately 60% of people infected with the Oropouche virus become symptomatic. The incubation period is typically 3–10 days. Providers should contact state, tribal, local, or territorial health departments to facilitate diagnostic testing.
As of August 2024, there are no approved Oropouche vaccines available.