Search API
The World Health Organization (WHO) announced today that three vaccines are available to prevent mpox in different countries.
Published on August 22, 2024, the WHO's Disease Outbreak News confirmed the MVA-BN® (JYNNEOS®, IMVAMUNE®), LC16-KMB, and OrthopoxVac are available in certain countries. However, OrthopoxVac has not yet been commercialized.
Based on extensive clinical research, the WHO recommends using MVA-BN or LC16 vaccines when the others are not available.
While the ACAM2000® live vaccinia virus vaccine is authorized to prevent mpox and smallpox infections, the WHO does not recommend it.
Furthermore, mpox vaccination is recommended by WHO and the U.S. CDC for individuals at high risk of exposure, such as when visiting mpox outbreak areas.
Takeda Canada Inc. today annonced a new report, Enhancing Diagnosis, Access, Care, and Treatment, highlighting the urgent need for innovative funding models and collaboration to help accelerate Canada’s National Strategy for Drugs for Rare Diseases.
Nearly 200 novel drugs for rare diseases are being developed and are expected to launch in Canada within the next ten years. It’s estimated only 5% of rare diseases have an approved treatment.
A “rare” disease is any disease that affects a minimal number of individuals. It is often genetic, chronic throughout a patient’s life, and life-threatening. With rare diseases affecting relatively limited patients, innovative treatments are often unavailable.
The impact of rare diseases is significant, with approximately one in 12 Canadians, two-thirds of whom are children.
“Canadians living with rare diseases have every reason to be optimistic,” says Durhane Wong-Rieger, President & CEO of the Canadian Organization for Rare Disorders, in a press release on August 22, 2024.
“Hundreds of new therapies are being developed, many targeting the 95% of rare diseases with no known treatment! We must leverage the $1.5 billion Rare Disease Drug Strategy,
The journey toward appropriately managing a rare disease is long and challenging. On average, it takes 6 to 8 years before a patient receives a correct diagnosis; this time, they will see an average of eight physicians and receive two to three misdiagnoses.
Takeda also produces innovative products, such as QDENGA®, an approved two-dose vaccine that prevents dengue fever and/or severe dengue in adults caused by any of the four serotypes of the dengue virus.
This dengue vaccine is authorized in about 40 countries and does not require pre-admission testing.

The United States Agency for International Development (USAID) announced up to an additional $35 million in emergency health assistance to bolster response efforts for the clade Ib mpox outbreak in Central and Eastern Africa, pending U.S. Congressional Notification.
This new commitment on August 20, 2024, brings the total U.S. government support for the affected countries in the region to more than $55 million in response to the ongoing mpox outbreak.
USAID support includes assistance with surveillance, diagnostics, risk communication, community engagement, infection prevention and control, case management, and vaccination planning and coordination.
The USAID support includes donating 50,000 doses of the third-generation JYNNEOS® (MVA-BN®, IMVAMUNE®) mpox / smallpox vaccine to the Democratic Republic of the Congo (DRC), the country most severely impacted by the outbreak.
Since 2023, this mpox outbreak has extended beyond the DRC, with several other countries in the region reporting cases in 2024, including countries where mpox has historically not been reported.
The current mpox outbreak differs in disease severity from the global clade II outbreak that began in May 2022, impacting the United States.
According to real-world evidence published in The Lancet Infectious Diseases today, this analysis is the first to provide estimates of Merck's Ervebo® (rVSV-ZEBOV) vaccine against Zaire Ebolavirus disease amid the widespread use of the vaccine during a large outbreak.
Announced on August 20, 2024, these findings confirm that Ervebo is highly protective against 84% (95% credible interval, 70% to 92%) of Ebolavirus disease and supports its use during outbreaks, even in challenging contexts such as in the eastern Democratic Republic of the Congo (DRC).
This finding is essential since Ebolaviruses are endemic in the DRC.
In a related Editorial, the authors wrote the 2018–20 Ebola virus disease epidemic in the DRC resulted in 3,470 reported cases and remains the second-largest Ebolavirus outbreak in recorded history worldwide. The initial Ebola outbreak was in 1976.
In November 2019, the World Health Organization prequalified the Ervebo vaccine. The U.S. Food and Drug Administration approved it on December 19, 2019.
Médecins Sans Frontières (Doctors Without Borders) funded this study.
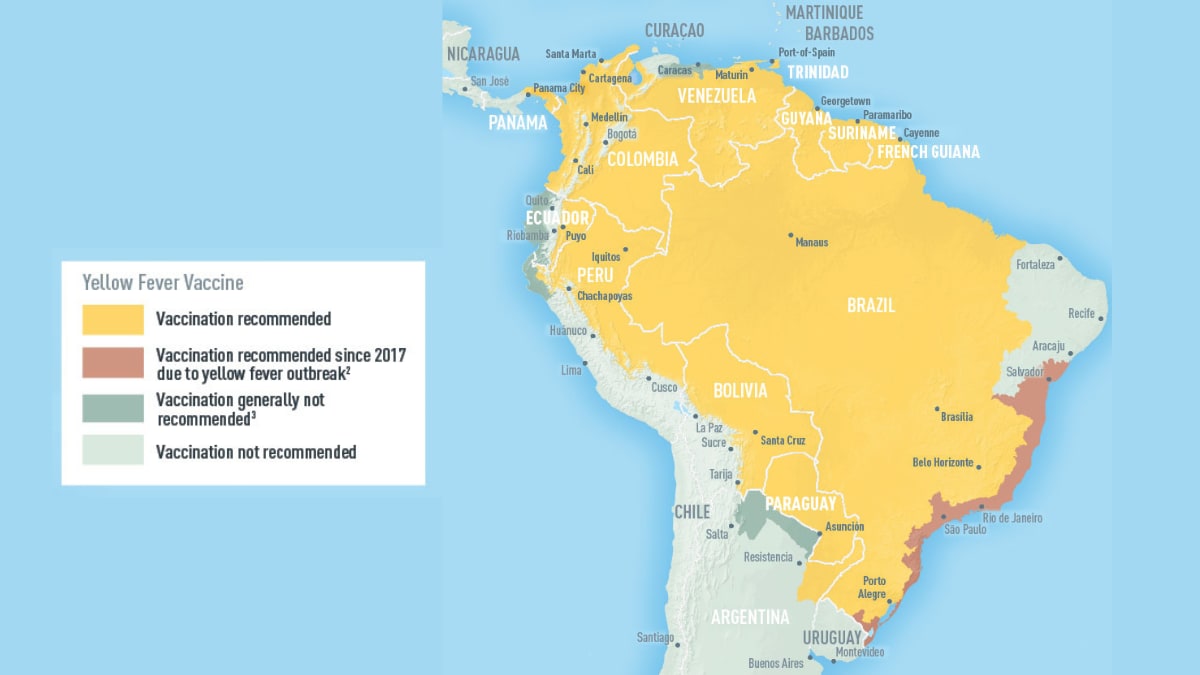
In 2024, yellow fever outbreaks remain a health threat in tropical regions of Africa and South America. The good news is that vaccines have been proven safe and effective for protecting international travelers visiting these areas.
However, new yellow fever vaccines with improved production scalability and enhanced efficacy are needed to reduce outbreaks.
The Lancet Infectious Diseases recently published results from a first-in-human phase 1 study on the safety and immunogenicity of a new Vero cell line-derived yellow fever vaccine, vYF-247.
Produced by Sanofi, the vYF-247 vaccine showed similar safety and immunogenicity to the U.S. FDA-approved YF-VAX vaccine.
These researchers concluded that the vYF-247 vaccine with a 5 Log CCID50 dose showed optimal viremia, safety, and immunogenicity and was chosen for further development.
Until a new vaccine is approved, the YF-VAX® vaccine remains available at travel clinics and pharmacies in the United States. For those travelers who were already vaccinated, the U.S. CDC says yellow fever vaccine booster doses are unnecessary.
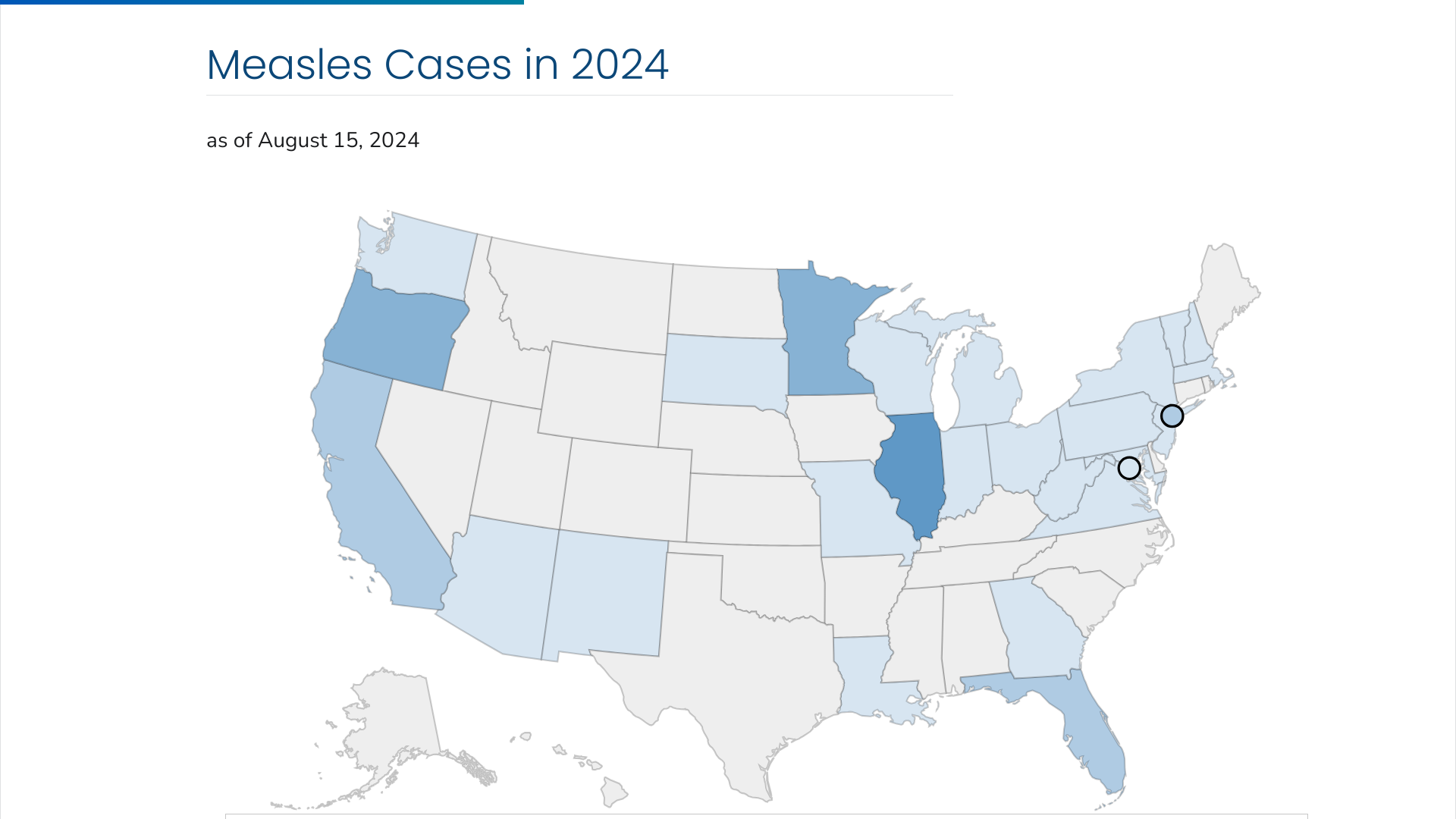
The Oregon Health Authority (OHA) has reported four new measles cases in 2024, bringing the total to 30 across three counties. Marion County has the most cases, followed by Clackamas County and Multnomah County.
As of August 21, 2024, all measles patients were unvaccinated, and twelve were younger than ten.
These counties and OHA have been sharing information with the public so “we can let members of the public know they may have been exposed to measles,” Clackamas County Health Officer Sarah Present, M.D. said in a recent press release.
Dr. Present noted that since measles is so contagious, an estimated 96% of the population needs to have received two doses of measles vaccine to protect the community's most vulnerable members via community or “herd” immunity.
In Oregon, measles vaccines are available at health clinics and local pharmacies.
As of August 15, 2024, the U.S. CDC confirmed that 27 U.S. jurisdictions reported 219 measles cases this year. In 2023, 20 jurisdictions reported 59 measles cases for the entire year.