Search API
ARS Pharmaceuticals, Inc. recently announced today that the European Commission (EC) has approved EURneffy® (adrenaline nasal spray) for the emergency treatment of allergic reactions for adults and children (≥30 kg) with severe allergies.
ARS Pharma anticipates that EURneffy will be made available in certain European Union Member States in Q4 2024. It is the first novel adrenaline delivery method approved in over three decades.
The pharmacodynamics and pharmacokinetics of 2 mg EURneffy were evaluated across a range of dosing conditions, including single and repeat dosing, self-administration by patients, dosing in pediatrics, and during multiple nasal conditions that can cause congestion and rhinorrhea such as nasal allergen challenge or infectious rhinitis caused by a cold/flu.
“Adrenaline is the only first-line treatment (not a preventive vaccine) for allergic reactions including anaphylaxis, yet there is significant underutilization of adrenaline due to the limitations of currently available therapy,” said Antonella Muraro, MD PhD, Professor of Food Allergy at the University of Padua, and lead author of the European Academy of Allergology and Clinical Immunology treatment guidelines for anaphylaxis, in a press release on August 26, 2024.
Type I severe allergic reactions are serious and potentially life-threatening events that can occur within minutes of exposure to an allergen and require immediate treatment with epinephrine, the only approved medication for these reactions in the European Union.
While adrenaline autoinjectors have been shown to be highly effective, well-published limitations result in many patients and caregivers delaying or not administering treatment in an emergency situation. These limitations include fear of the needle, lack of portability, needle-related safety concerns, lack of reliability, and complexity of the devices. Even if patients or caregivers carry an autoinjector, more than half either delay or do not administer the device when needed in an emergency, says the company.
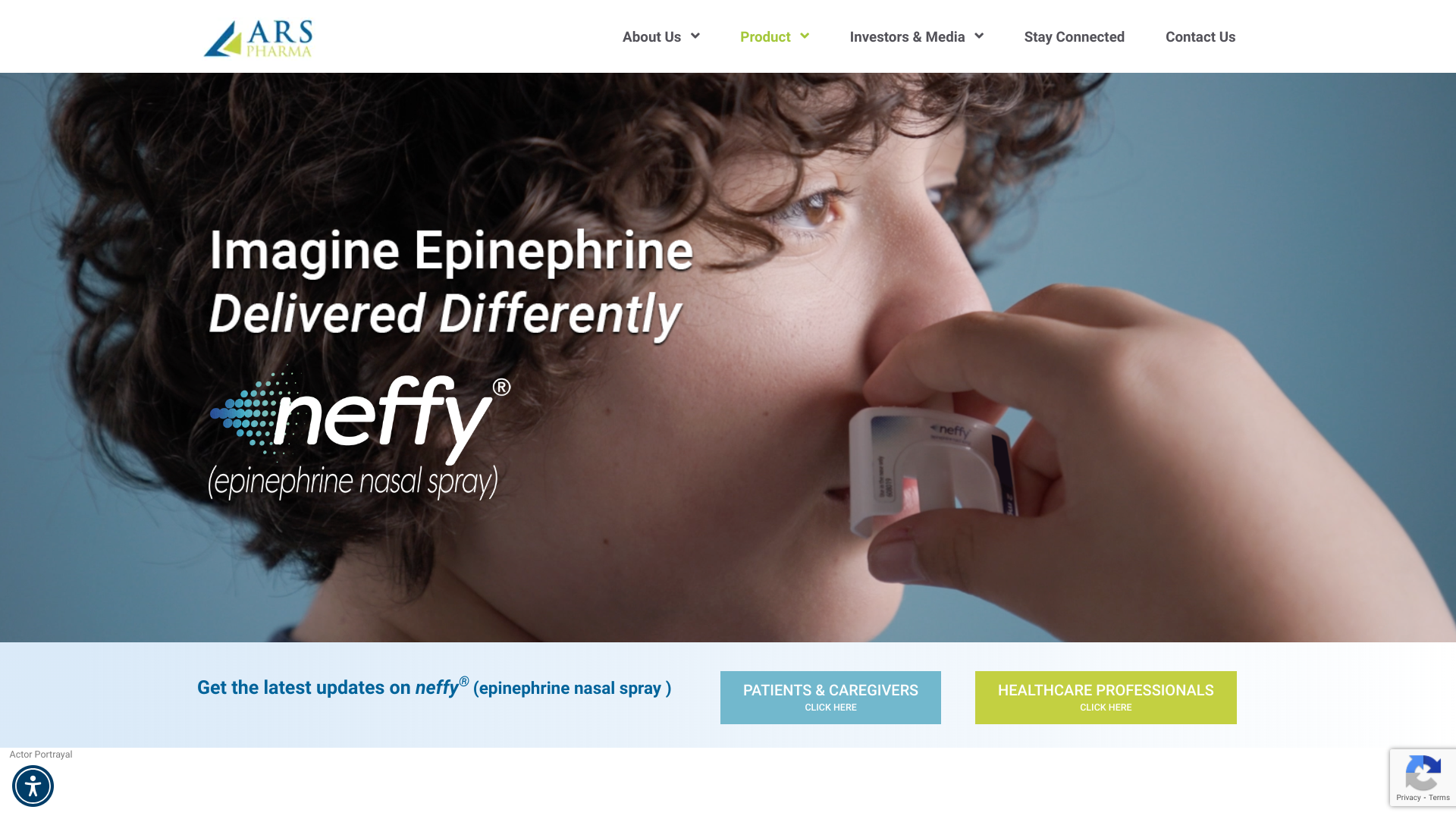
The U.S. CDC's Early Release Morbidity and Mortality Weekly Report on August 27, 2024, revealed that 21 Oropouche virus disease cases among U.S. travelers returning from Cuba have been reported this year.
At least three patients had recurrent symptoms after the initial illness, a common characteristic of Oropouche virus disease.
Most of these cases (20) were people in Florida.
The CDC recently published a Level 1 Travel Health Notice to alert travelers regarding Oropouche outbreaks in the Americas Region and Europe (19).
From December 2023 to June 2024, large Oropouche virus disease outbreaks were recognized in areas with known endemic diseases, and the virus emerged in new places in South America and Cuba where it had not been historically reported.
This year, cases have been reported in Bolivia, Brazil, Colombia, Cuba, and Peru.
From 2015 to 2022, only 261 cases of Oropouche fever were recorded in Brazil. However, as of August 6, 2024, Brazil confirmed 7,497 cases. However, the infectious rate has recently diminished.
The CDC says clinicians and public health jurisdictions should be aware of Oropouche virus disease in U.S. travelers and request testing for suspected cases.
In Florida, the Department of Health identified suspected Oropouche cases primarily by reviewing patients who received negative dengue test results and had visited countries such as Cuba.
Reported symptoms commenced during May–July and most commonly included fever (95%), myalgia, headache, fatigue or malaise, and arthralgia.
Travelers should prevent insect bites when traveling, and pregnant persons should consider deferring travel to areas experiencing outbreaks of Oropouche virus disease, says the CDC.
As of August 28, 2024, there are no approved vaccines to prevent Oropouche virus disease.
UCLA Health recently announced it had received a $120 million commitment from surgeon, inventor, and philanthropist Dr. Gary Michelson and his wife, Alya, to kick-start the California Institute for Immunology and Immunotherapy, an innovative public-private partnership aimed at spurring breakthrough discoveries that prevent and cure diseases and catalyze economic growth and innovation in Los Angeles.
Announced on August 27, 2024, the gift will be distributed via the Michelson Medical Research Foundation, designates $100 million to establish two research entities within the institute, each funded by $50 million,
One entity will focus on rapid vaccine development and the other on harnessing the microbiome to advance human health. The microbiome research will be conducted with the new UCLA Goodman-Luskin Microbiome Center.
In addition, the foundation, a part of the Michelson Philanthropies network of foundations, is funding a $20 million endowment to provide research grants to young scientists using novel processes to advance immunotherapy research, human immunology, and vaccine discovery.
“Immunology is the mediator of nearly all human diseases, whether we’re talking about cancer, heart disease, or Alzheimer’s,” Dr. Michelson said in a press release.
“The vision for this institute is to become a ‘field of dreams’ — the world’s leading center for the study of the immune system to develop advanced immunotherapies to prevent, treat, and cure all of the diseases that afflict people today and to end these diseases in our lifetime.
“Scientific research is the key to making possible longer and healthier lives,” Michelson added.

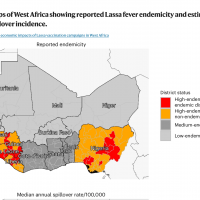
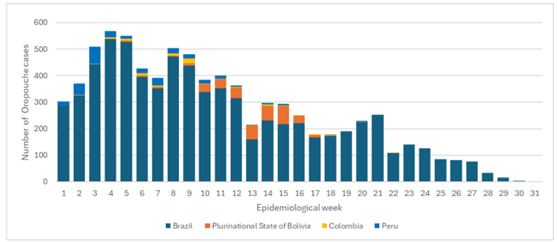
The International AIDS Vaccine Initiative (IAVI) recently announced that clinical trial sites in the Lassa fever-endemic countries of Ghana, Liberia, and Nigeria were vaccinating volunteers in IAVI's C105 study of a Lassa fever vaccine candidate.
This study is designed to evaluate the vaccine candidate’s safety, tolerability, and immunogenicity at two different dosage levels in adults, including people living with HIV, as well as in adolescents and children two years of age and older.
The IAVI C105 study results are expected in 2025. Should the vaccine candidate be found safe and efficacious, IAVI is committed to making its Lassa vaccine affordable and accessible to all needy populations.
As of August 28, 2024, no Lassa fever vaccine currently exists. However, several vaccine candidates are conducting research.
Lassa virus (LASV) is a zoonotic disease that causes the acute viral hemorrhagic illness called Lassa fever, for which treatment is limited.
People can get Lassa fever by contacting infected rats or their saliva, urine, or droppings. The U.S. CDC says that LASV can spread among people.
About 300,000 people fall ill across West Africa annually, though the actual disease burden is thought to be much higher. For these reasons, Lassa fever is featured in the World Health Organization’s R&D Blueprint and requires urgent action due to its potential to cause an outbreak of international concern.
Bharat Biotech International Limited (BBIL) announced on X the launch of HILLCHOL (BBV131), a novel single-strain Oral Cholera Vaccine (OCV).
Like other OCVs, HILLCHOL is a two-dose vaccine, which BBIL says needs to be orally administered on Day 0 and Day 14. The vaccine is suitable for individuals aged above one year.
However, it differs from the other vaccines because it is a single-strain OCV. BBIL stated on August 27, 2024, that this feature enhances manufacturing ease and efficacy.
As of August 2024, there is a global shortage of OCVs.
BBIL's collaboration with MSDInvents, Wellcome Trust, and Hilleman Laboratories aims to address the critical shortage of OCVs.
Currently available OCVs include Valneva SE's DUKORAL®.

Even though we cannot predict what will happen in the United States this upcoming flu season, by examining trends observed in the Southern Hemisphere this past season, we can gain valuable insights into what activity might occur during the forthcoming 2024-2025 Northern Hemisphere flu season.
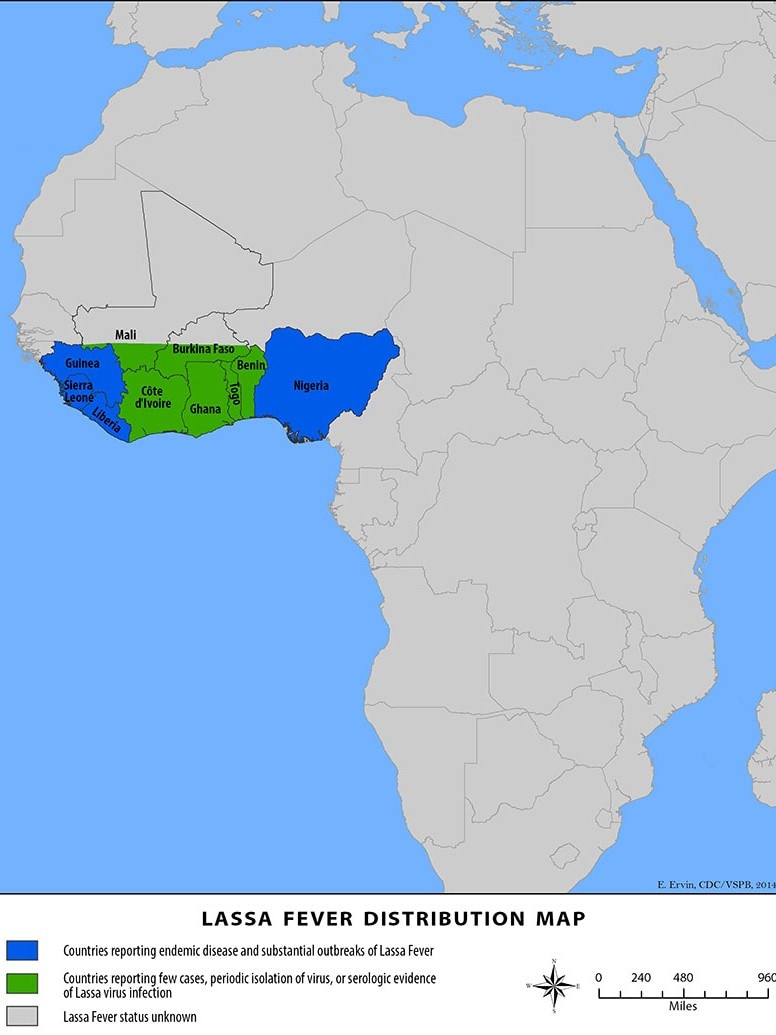
The WHO's Global Respiratory Virus Activity Weekly Update N° 489 was posted on August 11, 2024, confirming in the Southern hemisphere, influenza activity remained elevated in countries in South America (due to influenza A(H3N2) and B viruses), Eastern Africa (due to A(H1N1)pdm09 viruses), Southern Africa (due to B viruses), and Oceania (due to A(H3N2) viruses).
According to the U.S. Centers for Disease Control and Prevention (CDC), on August 26, 2024, during the 2024 Southern Hemisphere flu season, most countries experienced similar levels of flu activity compared to trends observed in prior seasons (2017‒2019 and 2022‒2023 flu seasons).
However, South American and Southern African countries experienced high influenza virus detection levels.
"Vaccination remains the best defense against flu, and even if vaccination does not entirely prevent the risk of flu, it can help reduce the severity of flu illness in people who get flu despite being vaccinated," the CDC wrote.
"In the U.S., September and October are generally good times to be vaccinated against flu."
As of late August 2024, various influenza vaccines are available at health clinics and pharmacies in the U.S.
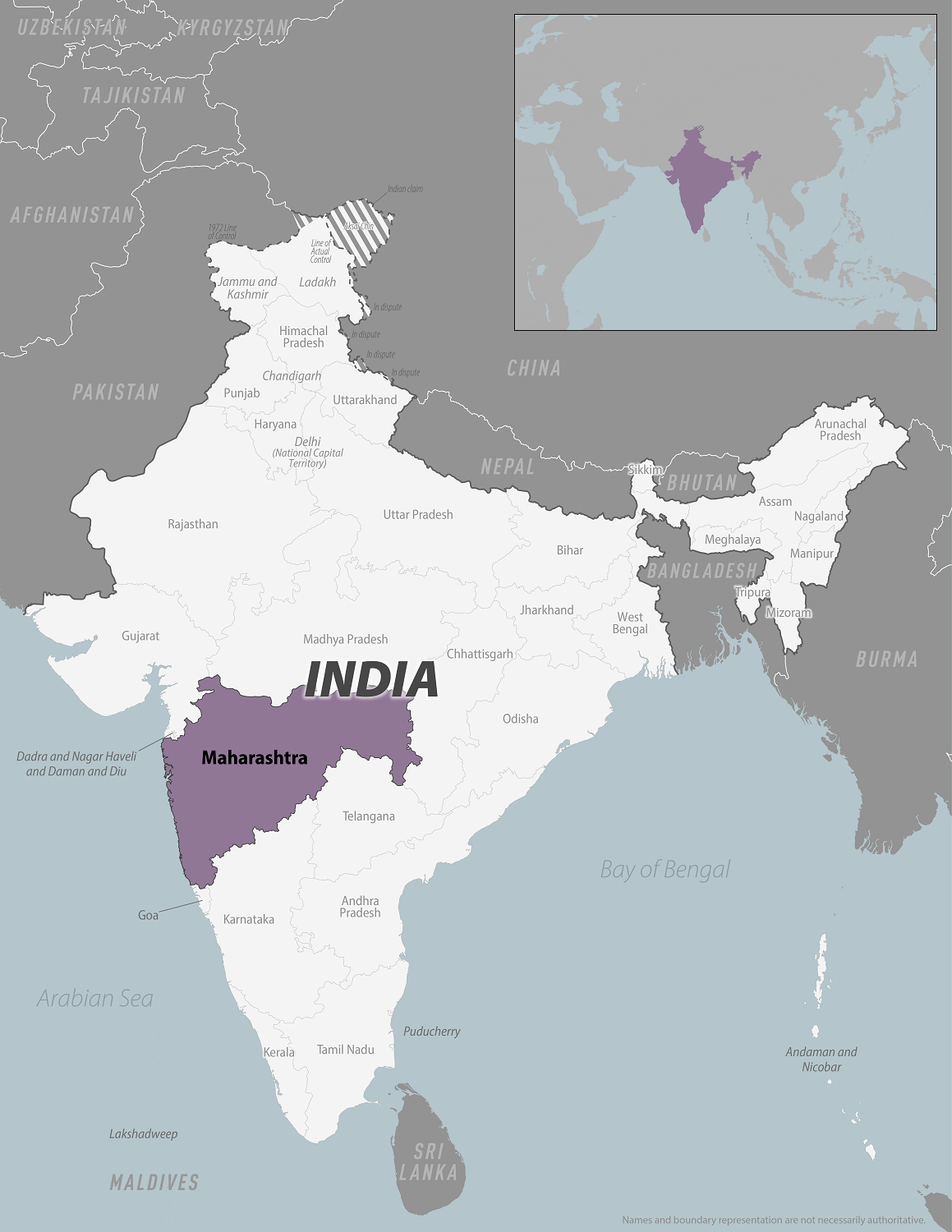
The U.S. Centers for Disease Control and Prevention (CDC) recently confirmed there is an outbreak of Zika virus in the state of Maharashtra, India.
The Times of India recently reported 113 confirmed Zika cases, of which 100 are from Pune district, including pregnant women.
On August 22, 2024, the CDC published a Level 2 - Practice Enhanced Precautions, Travel Health Notice, offering specific Zika advice.
The CDC says all travelers to Maharashtra should prevent mosquito bites and sexual transmission of the Zika virus during and after travel. Zika virus is most commonly spread to people by the bite of an infected Aedes species mosquito.
If you are planning pregnancy, you should delay pregnancy following travel to India based on the timeframes to prevent sexual transmission.
If you are pregnant, you should avoid travel to Maharashtra. If travel is unavoidable, you should strictly follow Zika prevention recommendations from your healthcare provider.
Infection during pregnancy can cause certain birth defects, says the CDC.
Furthermore, travelers to Maharashtra should seek medical care immediately if they develop fever, rash, headache, joint or muscle pain, or red eyes during or after travel.
In addition to India, there have been over 40,000 Zika cases confirmed in the Region of the Americas in 2024.
There is currently no vaccine to prevent a Zika infection.
However, Valneva SE's VLA1601 second-generation Zika vaccine candidate has progressed in clinical trials.