Search API
A new study from researchers at Wake Forest University School of Medicine sheds light on how the U.S. news media recently portrayed scientific evidence and the uncertainty surrounding unproven therapeutics.
The research team analyzed news reports on how scientific evidence, evidence details and limitations, safety, efficacy, and sources of authority were portrayed to the public.
“We found that 67% of news reports included scientific evidence, but only 24% mentioned scientific publications or journals,” said the study’s corresponding author in a press release on August 29, 2024.
Zubin Master, Ph. D., associate professor of social sciences and health policy at Wake Forest University School of Medicine, commented, “This period of time (the recent pandemic) was when medical specialists and the general public were anxiously scrambling to learn as much as possible about prevention and treatments because there were yet no proven therapeutics or vaccines."
"This makes for an ideal case study to examine how the news media portrays scientific evidence.”
According to the American Press Institute, only 40% of the public read news articles beyond headlines or lead paragraphs.
“It’s crucial, especially with controversial science topics, that the evidence and uncertainty are featured more prominently,” Master said.
The study authors also noted that science can be strengthened by acknowledging limitations and by portraying science as a process that is constantly changing and being corrected as additional knowledge is gained.
These findings appear online in the Journal of Medical Internet Research Infodemiology.

With more than 150 countries and territories reporting rabies cases, accessing one of the four authorized vaccines is essential to reducing this viral disease, says the World Health Organization.
Bavarian Nordic recently announced it is in agreement with the U.K.'s Medicines and Healthcare products Regulatory Agency (MHRA) regarding reports of rubber particles after reconstitution of the Rabipur rabies vaccine.
Bavarian Nordic has recently received an unexpected number of product quality complaints about visual particles in the vaccine solution.
Recommendations, issued on August 14, 2024, have been provided to minimize health risks. The analysis revealed that these particles consisted of rubber transferred from the rubber stopper of the vaccine vials during (coring) reconstitution.
The MHRA's letter to healthcare providers recommends that the reconstituted Rabipur vaccine be carefully inspected visually and not administered if visible particles are present. Suspected adverse drug reactions can be reported to the MHRA through the Yellow Card scheme.
While most of the world traces rabies infections in people to bites from an infected dog, in the United States, rabid bats are the leading source of infection.

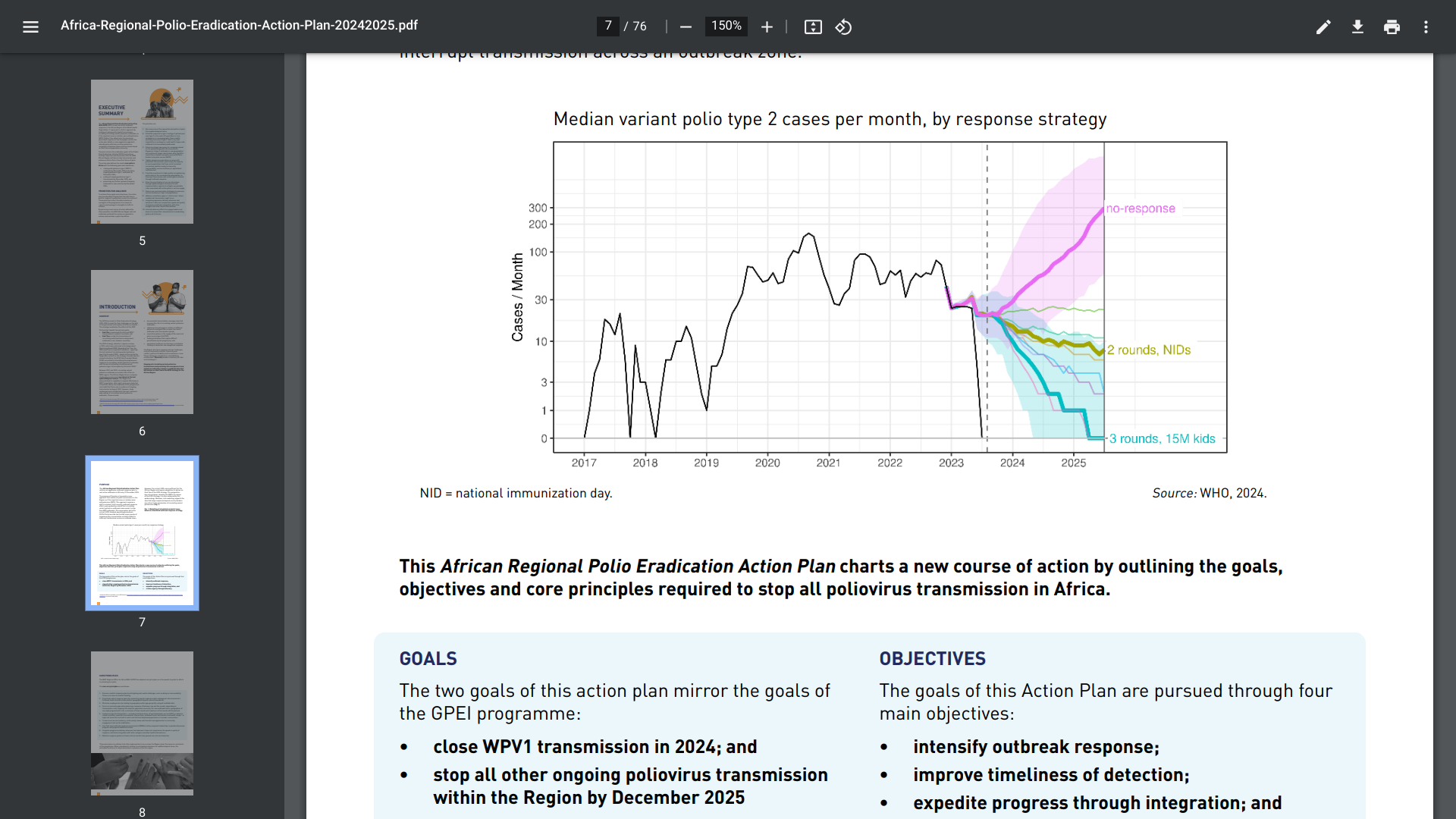
The recently announced Africa Regional Polio Eradication Action Plan 2024-2025 outlines a new strategy for responding to polio outbreaks.
Released on August 19, 2024, the pre-publication version introduces a different approach by addressing all instances of poliomyelitis (polio) transmission, including outbreaks of circulating variant poliovirus, as if they were cases of wild poliovirus (WPV).
This action plan advocates for a more proactive strategy instead of following the previous two-round campaign response. It suggests that countries affected by polio should conduct between three and five vaccination campaign rounds based on their specific risk and population immunity.
The plan's authors wrote, 'By pursuing a new course of action defined by these priorities, the WHO African Region will end outbreaks and build the resilience required to achieve and maintain a polio-free Africa.'
Furthermore, this plan offers hope for the countries currently reporting poliovirus detections. On August 28, 2024, the Global Polio Eradication Initiative reported the following summary:
Afghanistan: four WPV1 cases,
Pakistan: two WPV1 cases and 16 positive environmental samples,
Chad: two cVDPV2 cases and two positive environmental samples,
Côte d’Ivoire: two cVDPV2 positive environmental samples,
DR Congo: one cVDPV2 case,
Niger: one cVDPV2 positive environmental sample,
Nigeria: three cVDPV2 cases and one positive environmental sample,
Palestinian territory: one cVDPV2 case,
South Sudan: one cVDPV2 case.
To alert international travelers to their potential polio risk, the U.S. CDC reconfirmed in August 2024 that before any of 37 countries, ensure you are up to date on your polio vaccines.
The GPEI previously launched its Polio Eradication Strategy 2022–2026. The GPEI strategy called for a rigorous review of its plan in 2023.
As of late August 2024, the type 2 novel oral polio (nOPV2) vaccine, produced by PT Biofarma, has been administered over one billion times in various countries. This vaccine was designed to improve phenotypic stability and make the poliovirus strains less prone to reversion to virulence.
UNICEF today announced that it had issued an emergency tender for the procurement of mpox vaccines. The emergency tender is designed to secure immediate access to available mpox vaccines and expand production.
Depending on demand, manufacturers' production capacity, and funding, agreements for up to 12 million doses through 2025 can be made.
Based on recent announcements, there are four mpox vaccines available in 2024.
“Addressing the current mpox vaccine shortage and delivering vaccines to communities who need them now is of paramount importance. There is also a pressing need for a universal and transparent allocation mechanism to ensure equitable access to mpox vaccines,” said Director of UNICEF Supply Division Leila Pakkala in a press release on August 31, 2024.
UNICEF is the world’s largest single vaccine buyer, procuring more than 2 billion doses of vaccines annually for routine child immunization and outbreak response on behalf of nearly 100 countries.

The U.S. Food and Drug Administration today announced it granted emergency use authorization (EUA) for an updated version of the Novavax COVID-19 vaccine that more closely targets currently circulating SARS-CoV-2 virus variants to provide better protection against serious consequences of COVID-19, including hospitalization and death.
Novavax's updated COVID-19 vaccine targets the "parent strain" of KP.2 and KP.3, formulated to target the JN.1 variant.
The updated monovalent, protein-based vaccine is authorized for individuals 12 and older.
This FDA approval helps protect Americans and their families during the U.S.'s biggest surge in COVID-19 cases since January 2022.
The FDA has determined that the updated Novavax COVID-19 vaccine has met the statutory criteria for issuance of an EUA, including that the known and potential benefits of the vaccine outweigh its known and potential risks.
Peter Marks, M.D., Ph.D., director of the FDA’s Center for Biologics Evaluation and Research, commented in a press release on August 30, 2024, “Today’s authorization provides an additional COVID-19 vaccine option that meets the FDA’s standards for safety, effectiveness and manufacturing quality needed to support emergency use authorization.”
Following the Center for Biologics Evaluation and Research's release of vaccine batches, Novacax vaccines will be available in thousands of locations, including retail and independent pharmacies and regional grocers.

Emergent BioSolutions Inc. today announced that the U.S. Food and Drug Administration (FDA) approved the supplemental Biologics License Application for the expansion of the indication for the single-dose ACAM2000® Smallpox vaccine to include the prevention of mpox disease in individuals determined to be at high risk for mpox infection.
ACAM2000® is administered percutaneously via a bifurcated needle dipped into the vaccine solution. The skin is pricked several times in the upper arm with a droplet of the vaccine.
ACAM2000 was first approved by the FDA in 2007.
Dr. Amesh A. Adalja, FIDSA FACP FACEP & health security and emerging infectious diseases expert, Johns Hopkins Center for Health Security, commented in a press release on August 29, 2024, “ACAM2000®, a direct descendant of the Jenner vaccine (humanity’s first) which was used to eradicate smallpox, and now with the broadened indication, will be an invaluable tool in this endeavor.”
The FDA's labeling for ACAM2000® contains a contraindication for individuals with severe immunodeficiency. Severe localized or systemic infection with vaccinia (progressive vaccinia) may occur in persons with weakened immune systems. Individuals with severe immunodeficiency who are not expected to benefit from the vaccine should not receive ACAM2000®.
The risk of experiencing severe vaccination complications must be weighed against the risk of experiencing a potentially severe or fatal smallpox or mpox infection.
Additionally, there are warnings and precautions for myocarditis, pericarditis, encephalitis, encephalomyelitis, encephalopathy, progressive vaccinia, generalized vaccinia, severe vaccinial skin infections, erythema multiforme major (including Stevens-Johnson Syndrome), eczema vaccinatum resulting in permanent sequelae or death, accidental eye infection (ocular vaccinia), which can cause ocular complications that may lead to blindness, and fetal death.
These side effects may occur following primary or revaccination with live vaccinia virus vaccines, including ACAM2000®. These risks are increased in certain individuals and may result in severe disability, permanent neurological sequelae, and/or death.
As of late August 2024, four mpox vaccines are in use globally.

Health officials in Minnesota today announced they are urging families to ensure they are up to date on their measles, mumps, and rubella (MMR) vaccine as the four-month measles outbreak continues to impact the Twin Cities metro area.
Since May 2024, 30 cases of measles have been reported in Minnesota. So far, the outbreak is mainly affecting unvaccinated children in the Somali community in Minnesota.
In 2023, the Minnesota Department of Health (MDH) did not report a local measles case.
MDH says most measles cases in Minnesota result from people traveling to or from countries where measles is common and who become infectious with measles after arriving in Minnesota.
“Measles is currently circulating, and infections can be severe,” said Dr. Ruth Lynfield, state epidemiologist and medical director at the MDH, in a press release on August 28, 2024.
“I urge all parents to vaccinate their children because we know that vaccination offers the best protection.”
In Minnesota, the Vaccines for Children program (MnVFC) provides Free or Low-cost Vaccines for Children who are uninsured, enrolled in a Minnesota health care program like Medical Assistance or MinnesotaCare, or American Indian or Alaska Native.
Over 750 healthcare providers, including pharmacists, are enrolled in MnVFC, and approximately half of Minnesotan children are eligible.
Additionally, children who do not have health insurance to cover vaccines can also get vaccinated through local public health departments. Check your county government website to find a local public health immunization clinic.
Minnesota is not the only state reporting a measles outbreak in 2024. On August 22, 2024, the U.S. CDC reported 227 measles cases in 29 jurisdictions, led by Chicago, Illinois.