Search API
With the 2024-2025 Respiratory Syncytial Virus (RSV) season detected in Florida, recent research confirms the newly approved passive immunization monoclonal antibody offers infants significant protection.
On September 11, 2024, a study conducted in Spain and published by the American Academy of Pediatric Association concluded that Beyfortus™ (Nirsevimab) could effectively protect a broad infant population against RSV infection: a 63.1% reduction in acute bronchiolitis-related hospital admissions (95% confidence interval [CI], 60.9% to 65.2%) and a 63.1% reduction in pediatric intensive care unit admissions (95% CI, 58.1% to 67.9%).
In clinical trials, Beyfortus was reported to be about 90% (95% CI = 75%–96%) protective against RSV-associated hospitalization in infants in their first RSV season.
As of March 2024, the U.S. CDC reported that among females with an infant under eight months old, 41.3% reported that their infant received Beyfortus.
Beyfortus is currently recommended for newborn children in Australia, Canada, China, Europe, Japan, and France.

According to an American Academy of Pediatrics study, the effectiveness of a single dose of the rotavirus vaccine against emergency department visits or hospitalizations for inflammation of the gastrointestinal tract was 78% in children younger than five years and 53% in older children.
On September 10, 2024, these researchers wrote rotavirus vaccines remain highly effective in preventing disease in children.
The U.S. Centers for Disease Control and Prevention says two types of rotavirus vaccine are available for infants, and both are safe and effective. Two or more doses of the rotavirus vaccine are recommended as the best way to protect against it.
The Vaccines for Children (VFC) Program allows children to access no-cost vaccines such as rotavirus. The VFC program helps families of eligible children who may not be able to afford or have access to vaccines.

Alopexx, Inc. today announced a collaboration with India-based Bharat Biotech for the co-development and commercialization of the broad-spectrum antimicrobial vaccine, AV0328.
AV0328 is a synthetic vaccine designed to target poly N-acetyl glucosamine (PNAG), a substance found on the surface of a wide range of bacterial, fungal, and parasitic pathogens.
In preclinical studies, targeting PNAG has shown effectiveness in preventing and treating infections caused by over 15 different pathogens.
A phase I, first-in-human clinical trial, has been completed, demonstrating that AV0328 is well-tolerated with no serious adverse events observed. The vaccine-induced antibodies are capable of killing a wide range of PNAG-expressing pathogens, reaffirming their potential as a broad-spectrum antimicrobial solution.
Dr. Krishna Ella, Executive Chairman of Bharat Biotech, commented in a press release on September 11, "024: We are proud to collaborate with Alopexx to bring AV0328 to the regions (India) where it is most needed."
"Our goal is to develop solutions to reduce antimicrobial resistance through vaccination. This collaboration aligns with our mission to provide safe, affordable, and high-quality vaccines to combat infectious diseases globally."
The companies will co-develop and commercialize AV0328 in India and other licensed territories as part of the collaboration.
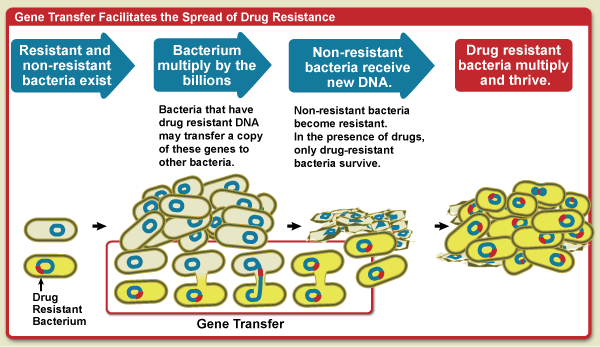
This Review discusses evidence that vaccines can play a major role in fighting antimicrobial resistance (AMR).
Different health organizations have highlighted the emergence and spread of AMR as a global threat. Pathogens resistant to AMR cause substantial morbidity and death. As resistance to multiple drugs increases, novel and effective therapies and prevention strategies are needed, wrote researchers in 2022.
Merck today announced positive top-line results from its pivotal Phase 3 clinical trial evaluating the company’s 9-valent Human Papillomavirus (HPV) vaccine, GARDASIL ® 9 vaccine, in Japanese males ages 16 to 26.
This trial (V503-064) met its primary and secondary endpoints demonstrating that administration of a 3-dose regimen of GARDASIL 9 reduced the combined incidence of anogenital persistent infection caused by nine types of HPV compared with a placebo.
“A decade after the first approval of GARDASIL 9, Merck continues to evaluate this important vaccine in additional patient populations and remains committed to helping prevent certain HPV-related cancers through broad and equitable access globally,” said Dr. Eliav Barr, senior vice president, head of global clinical development and chief medical officer, Merck Research Laboratories, in a press release on September 11, 2024.
“These data build on the clinical efficacy of GARDASIL 9 for the prevention of persistent infection in males and can potentially make a significant impact in addressing the global burden of certain HPV-related cancers and diseases.”
This HPV vaccine is generally available at clinics and pharmacies in the U.S. However, Merck says the GARDASIL 9 vaccination may not protect all vaccine recipients.

The Los Angeles County Department of Public Health (LACDPH) recently confirmed a locally acquired case of dengue fever in a person living in the City of Baldwin Park.
Dengue fever is transmitted primarily through the bite of infected Aedes mosquitoes.
As of September 9, 2024, this is the third locally acquired case of dengue fever in California and the first identified by the Los Angeles County Department of Public Health. Previous locally acquired cases of dengue fever in the greater LA area were identified in Long Beach and Pasadena in 2023.
The LACDPH stated the risk of transmission of dengue virus to the general population in Los Angeles County remains low.
As of September 5, 2024, the U.S. CDC reported that 53 jurisdictions, led by Florida, New Jersey, New York, and Puerto Rico, have reported 4,369 dengue cases this year. In 2023, 52 U.S. jurisdictions reported 2,343 dengue cases.
The CDC wrote that these local dengue cases reinforce the presence of infected mosquitoes and underscore the importance of preventive measures to control the spread of this virus.
As of September 10, 2024, dengue vaccines are not offered in the United States.

According to a new modeling study published by the Canadian Medical Association Journal, targeting respiratory syncytial virus (RSV) vaccinations to older adults (70+) with underlying health conditions is the most cost-effective way to reduce disease.
Published on September 9, 2024, this study found RSV vaccinations based on age plus risk for RSV-related complications were projected to avert a median of 20% to 31% of outpatient cases, 38% to 42% of hospital cases, and 39% to 42% of related fatalities.
“Strategies focused on adults with underlying medical conditions that place them at increased risk of RSV disease are more likely to be cost-effective than general age-based strategies,” wrote Dr. Ashleigh Tuite, the Centre for Immunization Programs at the Public Health Agency of Canada and the Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, with coauthors.
“We found that vaccination of older adults may be less costly and more effective than no vaccination and that vaccinating people aged 70 years and older with chronic medical conditions is likely to be cost-effective based on commonly used cost-effectiveness thresholds.”
Three approved RSV vaccines are available in the United States for the 2024-2025 season. These vaccines are generally available at clinics and pharmacies in the United States.

Evaxion Biotech A/S today announced new clinical phase 2 data for its lead compound EVX-01. The data show that 11 out of 16 patients had objective clinical responses, equaling a 69% Overall Response Rate, and 15 out of the 16 patients had reduced tumors.
This topline data is part of a one-year interim analysis of the ongoing phase 2 trial assessing EVX-01 in combination with Merck & Co., Inc. anti-PD-1 therapy, KEYTRUDA® (pembrolizumab) in patients with advanced melanoma.
EVX-01 is a personalized peptide-based neoantigen cancer vaccine/therapy (immunotherapy) for the first-line treatment of multiple advanced solid cancers. This combo therapy is tailored to target each patient's unique tumor profile and immune characteristics. It engages the patient’s immune system to fight off cancer by mounting a targeted response against tumors.
"A huge unmet medical needs remain in the field of melanoma, and we believe that EVX-01 could be an improved treatment option for patients. We look forward to presenting the complete one-year dataset at ESMO, discussing the data with potential partners, and advancing the phase 2 trial towards its completion next year,” says Christian Kanstrup, CEO of Evaxion, in a press release on September 9, 2024.
In the completed Phase 1/2a clinical trial, vaccine-induced T cells were detected in all patients.