Search API
As the global measles outbreak continues in 2024, a 32nd U.S. state confirmed its initial case.
The Tennessee Department of Health (TDH) recently confirmed that a Tennessee resident who recently traveled internationally is recovering from a measles infection.
As of September 14, 2024, TDH has not identified additional measles cases in Tennessee. The last year in which TDH reported positive measles cases in Tennessee was 2019.
On September 5, 2024, the U.S. CDC reported 251 measles cases in 31 jurisdictions this year. So far this year, Chicago (61), Minneapolis (49), and Portland (31) have reported an unusual number of cases.
The measles virus can spread through the air when an infected person speaks, coughs, or sneezes. It can live for up to two hours in the air or on a surface.
Symptoms include fever, headache, and general unwellness, followed by fever, rash, cough, red eyes, or congestion, says the TDH.
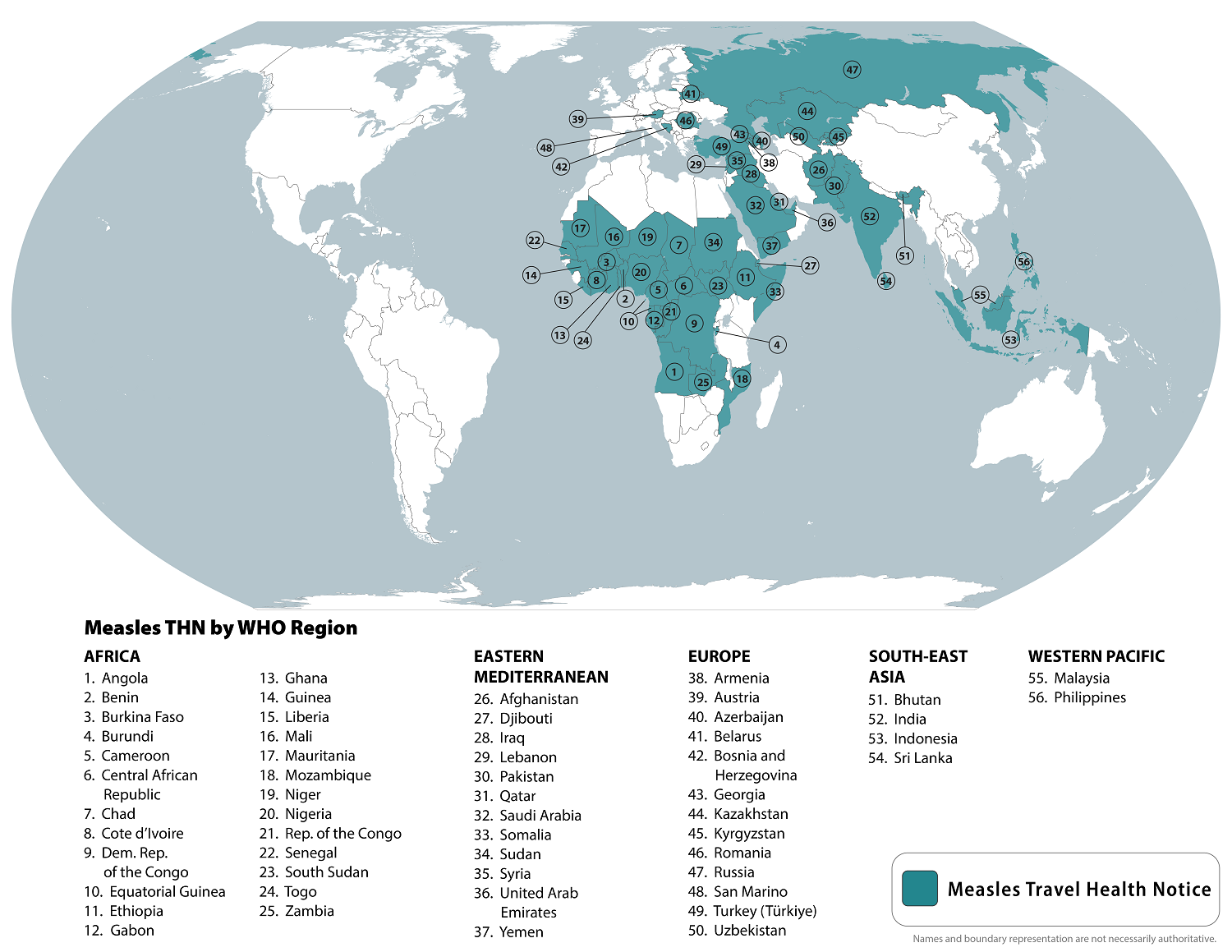
To notify international travelers of the measles outbreaks, the CDC reissued a Level Travel Health Advisory in August 2024, identifying 56 countries reporting measles cases to alert international travelers of the ongoing health risk.
The CDC recommends that international travelers speak with a healthcare provider at least one month before traveling abroad about measles vaccination options.
CSL and self-amplifying mRNA (sa-mRNA) pioneer Arcturus Therapeutics today announced that Japan's Ministry of Health, Labor and Welfare (MHLW) granted approval and authorization for their updated sa-mRNA COVID-19 vaccine, KOSTAIVE®.
According to the September 13, 2024, press release, KOSTAIVE is the world's first commercially available sa-mRNA COVID-19 vaccine for adults 18 and older.
Unlike conventional mRNA vaccines, sa-mRNA vaccines instruct the body to make more mRNA and protein to boost the immune response.
"We believe KOSTAIVE® has the potential to change the paradigm for COVID-19 vaccines in Japan," commented Jonathan Edelman, M.D., Senior Vice President, Vaccines Innovation Unit, CSL.
"Today's approval further demonstrates CSL's promise to pursue, develop, and deliver new innovative treatment options to protect public health."
Meiji Seika Pharma, CSL's exclusive partner in Japan, will begin vaccine distribution next month.
Following a peak in July 2024, MHLW data indicates COVID-19 cases in Japan have rapidly declined as of early September.
To avoid diseases such as Japanese encephalitis (IXIARO®), measles, and rubella, the U.S. CDC recommends speaking with a travel vaccine expert at least one month before visiting Japan.
CSL, including three businesses: CSL Behring, CSL Seqirus, and CSL Vifor, provides lifesaving products to patients in more than 100 countries and employs 32,000 people.
About two years ago, the World Health Organization (WHO) published a report highlighting the first-ever list of fungal priority pathogens, which included 19 fungi representing the greatest threat to public health.
Since then, the WHO has not recommended a fungal vaccine but continues to encourage the development of innovative therapies.
To address this unmet need, F2G Ltd announced a $100 million financing round that will enable the company to complete late-stage development, seek regulatory approval, and prepare for commercialization in the U.S. of olorofim, a novel oral antifungal therapy to treat invasive aspergillosis (IA) and other invasive fungal infections.
It is anticipated that this treatment will be used to treat patients with a serious invasive, rare fungal disease for which existing treatments are inappropriate or no longer effective.
Olorofim is the first orotomide antifungal, an entirely novel class of antifungal agents. It is the only antifungal medication awarded a Breakthrough Therapy Designation for multiple indications by the U.S. Food and Drug Administration.
Olorofim has a novel mechanism of action, different from that of existing classes of antifungals. It exerts fungicidal activity by inhibiting the pyrimidine synthesis pathway.
Henry Skinner, Ph.D., Chief Executive Officer of AMR Action Fund, commented in a press release on September 12, 2024, “Fungal infections are a growing threat to patients worldwide and have a disproportionate impact on vulnerable populations, yet there has been a paucity of innovation in the field of antifungals."
"For decades, clinicians have relied on a handful of antifungal classes, with few mechanisms of action and significant limitations due to spectrum of activity, drug toxicities, or drug-drug interactions. These therapies are increasingly failing in patients."
The U.S. NIH says some fungal infections are more common in people with weakened immune systems or hospitalized individuals, while others can infect anyone, including otherwise healthy people.
As of 2023, fungal vaccine candidates were segmented into broad categories based upon their composition, ranging from multiple to single antigens: whole organism vaccines (live-attenuated or killed fungal cells), crude extracts (fractions derived from cells and medium of fungal cultures), purified subunit vaccines (proteins, peptides), and nucleic acids (RNA and DNA) encoding the antigen(s) of interest.
No fungal vaccine has been approved as of 2024.
The U.S. Centers for Disease Control and Prevention (CDC) today published updated recommendations from the Advisory Committee on Immunization Practices (ACIP) regarding the use of 21-Valent Pneumococcal Conjugate Vaccine Among U.S. Adults.
On September 12, 2024, the CDC's MMWR confirmed the U.S. Food and Drug Administration approved 21-valent pneumococcal conjugate vaccine (PCV) (PCV21; CAPVAXIVE; Merck Sharp & Dohme, LLC) for adults aged ≥18 years.
PCV21 does not contain certain serotypes that are included in other licensed pneumococcal vaccines but adds eight new serotypes. It is recommended for all adults aged ≥65 years and adults aged 19–64 years with certain risk conditions for pneumococcal disease if they have not received a PCV or whose vaccination history is unknown.
Previously, options included either 20-valent PCV (PCV20; Prevnar20; Wyeth Pharmaceuticals, Inc.) alone or a 15-valent PCV (PCV15; VAXNEUVANCE; Merck Sharp & Dohme, LLC) in series with 23-valent pneumococcal polysaccharide vaccine (PPSV23; Pneumovax23; Merck Sharp & Dohme, LLC).
Additional recommendations for the use of PCV20 exist for adults who started their pneumococcal vaccination series with 13-valent PCV (PCV13; Prevnar13; Wyeth Pharmaceuticals, Inc.).
On June 27, 2024, ACIP recommended a single dose of PCV21 for adults aged ≥19 for whom PCV is currently recommended. Indications for PCV have not changed from previous recommendations.
This report summarizes the evidence considered for these recommendations and provides clinical guidance for using PCV21.
In the U.S., various healthcare providers offer pneumococcal vaccines.

Bavarian Nordic A/S today provided an update on its supply and manufacturing activities supporting the ongoing mpox outbreak. The Company produces MVA-BN® (JYNNEOS®, IMVAMUNE®, and IMVANEX®), a leading smallpox and mpox vaccine.
Since the recent declaration of a Public Health Emergency of International Concern (PHEIC) by the World Health Organization (WHO), Bavarian Nordic has intensified the collaboration with global stakeholders to support efforts to combat the mpox outbreak.
Thanks to donations from the European Commission, the U.S. government and Bavarian Nordic, the first doses of MVA-BN arrived last week in the Democratic Republic of Congo, the epicenter of the mpox clade 1b outbreak. More than 250,000 doses have already been shipped, and other countries have pledged further donations of more than 500,000 doses of MVA-BN.
Bavarian Nordic stated that by focusing the full capacity to address the current public health emergency, the Company could supply up to 13 million MVA-BN doses by the end of 2025, including 2 million in 2024.
Paul Chaplin, President & CEO of Bavarian Nordic, said in a press release on September 12, 2024, “As the supplier of the only non-replicating mpox vaccine that has shown to be highly effective during a mpox outbreak, we have been working closely with all governments and organizations to support the international efforts to combat the latest public health emergency."
"Just as during the clade two mpox outbreak in 2022/23, we will support all requests for vaccine and have already secured agreements and submitted responses to the UNICEF tender that will hopefully secure more access to MVA-BN globally."
The JYNNEOS vaccine is commercially available in the United States at select travel clinics and pharmacies.
The U.S. government recommends two JYNNEOS doses to provide robust protection against disease. However, routine immunization against mpox is not recommended for the general public, and a booster dose (3rd) is not endorsed.

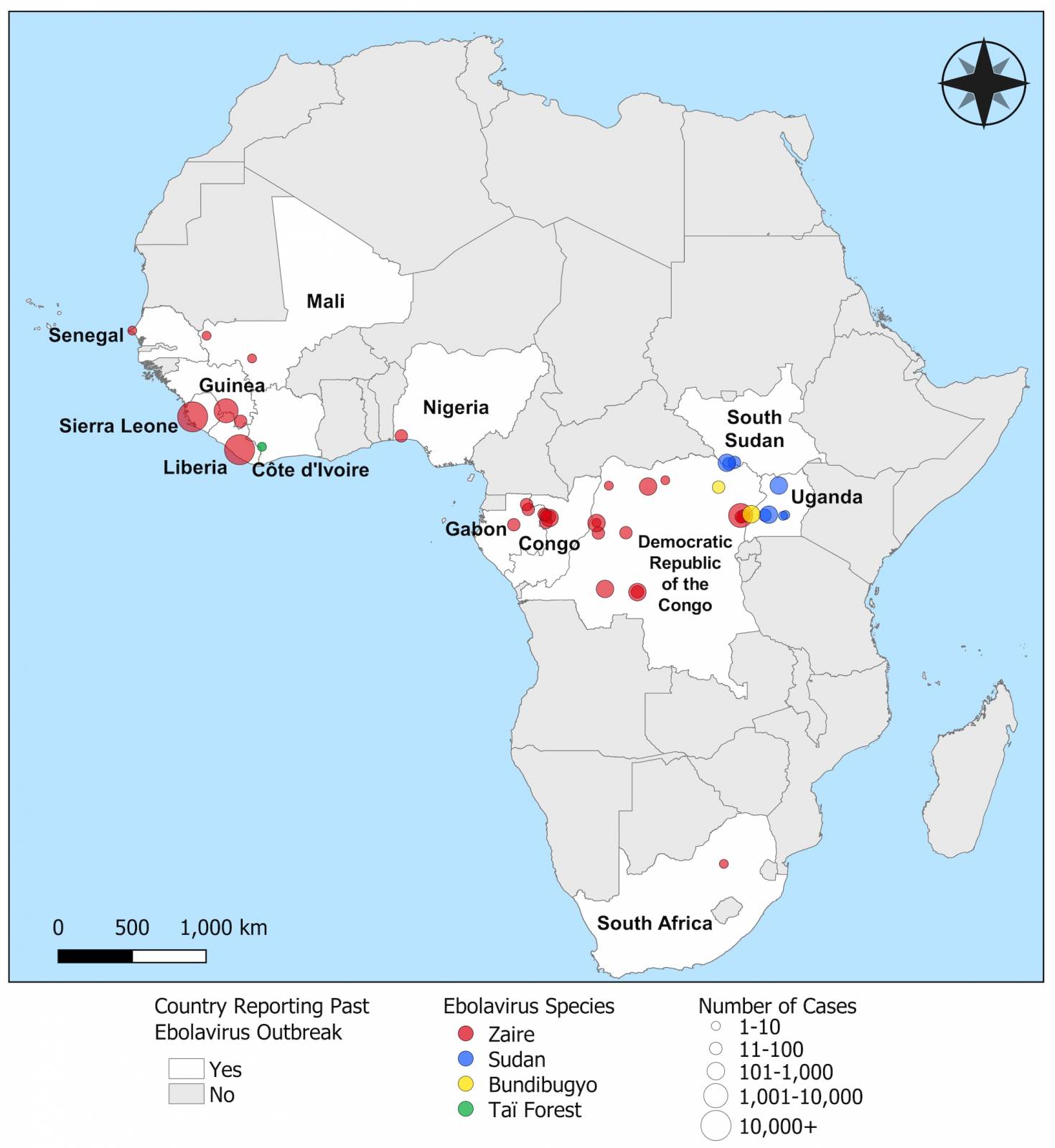
While there is an approved Ebola virus disease (EVD) vaccine, the U.S. government continues to invest in human monoclonal antibody (mAb) therapy during Zaire ebolavirus outbreaks in Africa.
The initial Zaire Ebolavirus disease (EVD) case was confirmed in 1976, Since then, more than 30 EVD outbreaks have been reported.
Emergent BioSolutions Inc. announced today that it was awarded a contract modification executing an option period by the Biomedical Advanced Research and Development Authority (BARDA), valued at $41.9 million, for drug substance engineering and scale-up process validation, long-term stability, and commercial readiness in support of its ongoing scale-up program for Ebanga™, a licensed glycoprotein (EBOV GP)-directed mAb treatment for EVD.
“Emergent is proud to continue to advance the Ebanga™ (ansuvimab-zykl) development and scale up to its next phase,” said Paul Williams, senior vice president of products business, Emergent, in a press release on September 12, 2024.
This mAb binds to a portion of the Ebola virus's surface called the glycoprotein, which prevents the virus from entering a person's cells. Ebanga's efficacy has not been established for other species of the Ebolavirus and Marburgvirus.
Under the terms of the contract, Emergent will complete activities to advance the development of Ebanga™ treatment through post-licensure commitments, including the transfer of technology as part of manufacturing scale-up, submission of a supplemental Biologics License Application to the U.S. Food and Drug Administration (FDA), and completion of stability studies.
The existing 10-year contract consists of a base period of performance with two option periods for advanced development valued at approximately $121 million and option periods for procurement of Ebanga™ treatment over five years valued at up to $583 million. Execution of this option period is in line with Emergent’s planned program performance and critical path for developing the Ebanga™ treatment.
BARDA is part of the Administration for Strategic Preparedness and Response within the U.S. Department of Health and Human Services.
Moderna, Inc., today announced program and financial updates at its annual R&D Day event, demonstrating progress and strategic prioritization of its mRNA pipeline.
Stéphane Bancel, CEO of Moderna, commented in a press release, "The size of our late-stage pipeline combined with the challenge of launching products means we must now focus on delivering these ten products to patients, slow down the pace of new R&D investment, and build our commercial business."
Moderna's updates on September 12, 2024, include but are not limited to, several seasonal influenza vaccine candidates in clinical development.
The Company's investigational seasonal flu vaccine, mRNA-1010, has demonstrated consistently acceptable safety and tolerability across three Phase 3 trials.
In the most recent Phase 3 trial (P303), which was designed to test the immunogenicity and safety of an optimized vaccine composition, mRNA-1010 met all immunogenicity primary endpoints, demonstrating higher antibody titers compared to a licensed standard-dose flu vaccine (Fluarix®).
In an older adult extension study of P303, mRNA-1010 met all primary immunogenicity, including superiority for all compared to a licensed enhanced flu vaccine (Fluzone HD®), and showed an acceptable reactogenicity profile.
The Company plans to start a confirmatory vaccine efficacy study for mRNA-1010 in 2024, funded by previously announced project financing through Blackstone Life Sciences.
Moderna also confirmed it is no longer pursuing an accelerated approval pathway for the regulatory submission of its standalone flu vaccine, mRNA-1010, to focus its resources on submitting a potentially more impactful flu/COVID combination vaccine, mRNA-1083, in 2024.
With the 2024-2025 flu season beginning in the United States, most health clinics and pharmacies offer flu shot vaccination services.
About 158 million flu vaccines had been distributed during the 2023-2024 season.



