Search API
The global dengue fever outbreak recently reached one of the most populated cities in the United States.
The Los Angeles County Department of Public Health announced it is investigating its third locally acquired dengue case in the City of Baldwin Park.
This is an unprecedented number of locally transmitted cases for a region of over 12 million people where mosquitoes have not previously transmitted dengue.
"The City of Baldwin Park is aware of the recent cases of locally acquired dengue in our community. While the risk of transmission remains low, we must take this situation seriously and act proactively," said Mayor Emmanuel J. Estrada, who serves as a trustee representing Baldwin Park on the San Gabriel Valley Mosquito and Vector Control District board, in a press release on September 18, 2024.
In 2024, the U.S. CDC confirmed that 50 jurisdictions, led by California, Florida, New Jersey, New York, and Puerto Rico, had reported 5,027 dengue cases as of September 20, 2024.
In the Region of the Americas, 43 countries and territories have reported over 11,712,499 dengue cases and 6,500 related deaths this year.
While dengue is a vaccine-preventable disease, no vaccine is currently available in the United States. However, Takeda's QDENGA® is an approved two-dose vaccine in about 40 countries.
GSK plc recently announced topline Phase 3 clinical trial data for a combination regimen of two of its vaccines: the RSV vaccine Arexvy and Shingrix, a market-leading shingles shot.
While GSK did not provide specific clinical trial (NCT05966090) data in its September 18, 2024 announcement, the company did confirm that co-administering Arexvy with Shingrix resulted in a “non-inferior immune response” compared with inoculation with the vaccines at separate visits.
The vaccine combo was also well-tolerated with an “acceptable” safety profile, according to GSK.
This is essential news as both RSV and shingles pose significant health risks to older adults, and these risks only increase with age as the immune system declines.
“With our co-administration studies, GSK is using its science and technology to help remove barriers to adult immunization, by potentially reducing the number of visits to the healthcare offices and pharmacies and ultimately help to get ahead of RSV and shingles,” Led Friedland, GSK’s vice president of scientific affairs and public health, said in the statement.
Results from this trial will be submitted for peer-reviewed scientific publication and used to support regulatory submissions to the U.S. FDA, the European Medicines Agency, and other regulators.

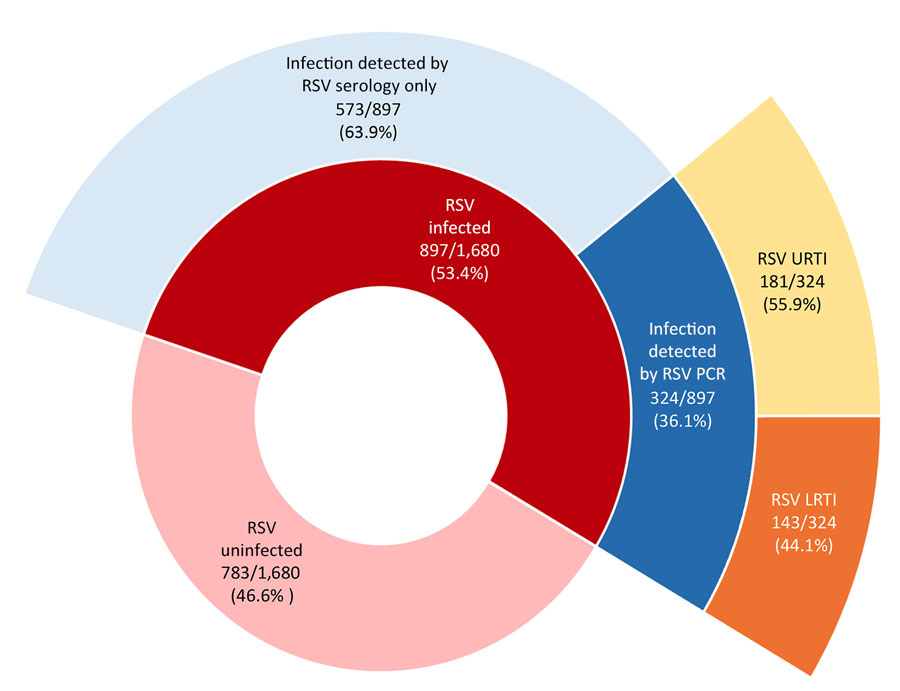
According to a Research Letter, Volume 30, Number 10—October 2024, published in Emerging Infectious Diseases, 53.4% of infants were infected with respiratory syncytial virus (RSV) during infancy, and 2.8% were hospitalized.
The Risk factors for RSV infection during infancy, in order of contribution, were:
- Infant birth month (June vs. referent October, OR 2.42 [95% CI 1.78–3.29]),
- Presence of siblings (OR 1.50 [95% CI 1.22–1.84]),
- Daycare attendance (OR 1.54 [95% CI 1.24–1.93]),
- Increasing percentage below the poverty level in the residential neighborhood (21% vs. 8%; OR 1.19 [95% CI 1.05–1.36]), and
- Public insurance (OR 1.28, 95% CI 1.02–1.62).
The researchers determined secondhand smoke exposure, sex, ever being breastfed, maternal asthma, and study year were not significantly associated with the likelihood of infant RSV infection.
In conclusion, 'our data are important estimates of RSV disease's burden and infection risk factors in healthy-term infants. Our findings provide a benchmark to monitor the effects of recently available maternal vaccines in the United States and extended half-life monoclonal antibodies (Beyfortus™) for preventing severe RSV illness in early life.'
As of 2023, the U.S. CDC says that infants and children who are recommended to receive Beyfortus should be immunized as quickly as possible.
According to the Times of India, 267 chikungunya cases were reported in Mumbai, India, between July and September 14, 2024. This data indicates India may exceed last year's 200,000 reported chikungunya cases.
BMC's health update on September 16, 2024, stated that doctors have confirmed that patients with mosquito-borne diseases, dengue, and chikungunya are being admitted to the Mumbai hospital with complaints of very high fever, vomiting, and severe body aches.
"Patients with chikungunya are literally bedridden due to debilitating joint pain and high temperatures,'' Dr Gautam Bhansali from Bombay Hospital informed the TOI.
The public health concern is that this viral disease may spread within Mumbai's 12 million residents.
Chikungunya disease was initially reported in India in 1963, and as of September 2024, every part of the country has become endemic.
The U.S. CDC stated in 2024 that the Chikungunya vaccination may be considered for certain visitors in India. The CDC recommends prospective travelers to India speak with a travel vaccine expert about Valneva SE's IXCHIQ® single-dose, live-attenuated chikungunya vaccine, at least one month before departure.
About 1.3 million U.S. travelers visited India last year.
IXCHIQ is available at various health clinics and pharmacies in the U.S.
Bavarian Nordic A/S and Gavi today announced an advance purchase agreement (APA) to secure 500,000 doses of the MVA-BN® mpox vaccine (JYNNEOS® or IMVANEX®).
These vaccines are being supplied to African countries impacted by the current mpox clade 1 outbreak.
Bavarian Nordic is ready to supply the mpox/smallpox vaccines pending signing a supply agreement with UNICEF, Gavi’s alliance partner, which will deliver these doses in 2024.
Paul Chaplin, President & CEO of Bavarian Nordic, commented in a press release on September 18, 2024, “The doses secured through this APA will significantly increase the availability of mpox vaccines for African countries, and we are pleased that Gavi has selected our MVA-BN vaccine, which has proven highly effective during the global mpox (clade 2) outbreak in 2022.”
The vaccines will be funded by Gavi, the Vaccine Alliance's First Response Fund, a new financial mechanism created in June 2024 to make cash available to purchase vaccines in health emergencies rapidly.
As of September 2024, there are four mpox vaccines available globally.

Based on today's announcement by Valneva SE, adolescents may soon have access to the only approved chikungunya vaccine. This is essential news as the chikungunya virus (CHIKV) has now been identified in over 110 countries in Asia, Africa, Europe, and the Americas.
On September 18, 2024, Valneva submitted label extension applications to the European Medicines Agency (EMA) and Health Canada to potentially expand the use of its approved chikungunya vaccine, IXCHIQ®, to adolescents aged 12 to 17 years in Europe and Canada.
The Canadian label extension application also includes two-year antibody persistence data, a key differentiator for IXCHIQ® that was already included in the initial EMA filing.
Valneva expects to submit data to the U.S. Food and Drug Administration (FDA) in 2024 to support potential label extensions in the U.S.
IXCHIQ is currently approved in the U.S., Europe, and Canada to prevent disease caused by mosquito-spreading CHIKV in individuals 18 and older.
In the U.S., the commercial launch is underway, as IXCHIQ is available at health clinics and pharmacies.
First sales in Canada and Europe are anticipated in the fourth quarter of 2024.
In addition to ramping up sales, Valneva is focused on expanding the vaccine’s label and access.
The Company expects a marketing authorization in Brazil in the second half of 2024 and recently expanded its partnership with The Coalition for Epidemic Preparedness Innovations (CEPI) to support broader access to the vaccine in Low Middle-Income Countries, post-marketing trials, and potential label extensions in children, adolescents, and pregnant women.
CEPI previously confirmed it will provide Valneva up to $41.3 million of additional funding over the next five years, with support from the European Union’s Horizon Europe program.
Juan Carlos Jaramillo, M.D., Chief Medical Officer of Valneva, commented in a press release, “Given the substantial risk that chikungunya presents to individuals residing in or traveling to endemic regions, it’s imperative to ensure the vaccine is available to all age groups."
"This broader accessibility would certainly help provide protection and mitigate the burden of this debilitating illness, which is currently spreading in areas that were previously unaffected."
"The durability of the immune response is also extremely important, especially for endemic countries where access to immunization can be difficult.”
EMA and Health Canada’s label extension applications are based on positive six-month adolescent Phase 3 data the Company reported in May 2024. These data showed that a single-dose vaccination with IXCHIQ® induces a high and sustained immune response in 99.1% of adolescents and that the vaccine was generally well tolerated.
The Lancet Infectious Diseases also recently published an article showing that the vaccine was well tolerated in adolescents aged 12 to 17 28 days after a single injection, regardless of previous CHIKV infection.
In addition to the adolescent data, Health Canada’s label extension application included IXCHIQ®‘s antibody persistence data, which showed that 97% of participants sustained the vaccine’s immune response for 24 months and was equally durable in younger and older adults.
Valneva expects to publish 36 months of persistence data later in 2024.