Search API
According to the U.S. government, malaria was eliminated from the United States in 1951, and almost all recent cases in the U.S. have been travel-associated.
As of the week ending October 19, 2024 (#42), the U.S. Centers for Disease Control and Prevention confirmed 1,576 travel-related malaria cases this year.
Most malaria cases are confirmed in New York, Florida, California, and Texas.
These cases were associated with travel to 85 countries where malaria remains endemic, such as Africa, and could represent a potential source of Plasmodium infection for locally acquired mosquito-transmitted cases in the U.S.
On October 24, 2024, the CDC reported (73(42);946–949) ten local malaria cases from May to September 2023. In Florida, seven locally acquired malaria cases were reported near Sarasota in 2023.
The CDC says that before traveling internationally to areas where malaria is endemic, travelers should consult with their healthcare provider regarding recommended malaria prevention measures to reduce personal and community risks.
While two malaria vaccines are WHO-recommended, neither are available in the U.S. in 2024.
During today's U.S. CDC's Advisory Committee on Immunization Practices (ACIP), Michael Melgar, M.D. presented a summary focused on a small number of Guillain-Barré syndrome (GBS) cases observed in clinical trials within 42 days after U.S. FDA-approved protein subunit RSV vaccinations (GSK Arexvy, Pfizer Abrysvo).
The ACIP Work Group concluded on October 24, 2024, that 'available data support the existence of an increased risk of GBS after protein subunit RSV vaccination. GBS risk following RSV vaccination is rare, with less than 10 cases per 1 million vaccinations.'
However, Moderna’s newly approved mResvia mRNA vaccine has not been associated with an increased risk of GBS. Post-licensure safety surveillance for mResvia began recently in June 2024.
Dr. Melgar's presentation stated, 'Due to the small number of cases (in older adults), it was unclear whether they represented a genuine association between RSV vaccination and GBS.'
The CDC says GBS syndrome happens when a person’s immune system harms their nerves. This harm causes muscle weakness and sometimes paralysis. CDC estimates that only about 3,000–6,000 people develop GBS annually in the United States.

GSK's Susan Gerber, M.D., Medical Director, today confirmed that about 9 million people in the United States have been vaccinated with AREXVY, a vaccine indicated for active immunization to prevent lower respiratory tract disease (LRTD) caused by respiratory syncytial virus (RSV).
During the CDC's Advisory Committee on Immunization Practices meeting on October 24, 2024, Dr. Gerber reaffirmed that protecting vulnerable individuals at high risk for severe RSV disease is essential ahead of this year's RSV season.
GSK also announced new preliminary data for AREXVY in adults aged 18-49 at increased risk for LRTD caused by RSV due to certain underlying medical conditions and in adults who are immunocompromised.
These data show the vaccine’s potential to help protect a broader group of adults at risk from the potentially serious consequences of RSV.
This U.S. FDA-approved RSV vaccine has been approved to prevent RSV-LRTD in individuals 60 years of age and older in more than 50 countries, including Europe and Japan.

The U.S. Centers for Disease Control and Prevention (CDC) Director today endorsed the Advisory Committee on Immunization Practices (ACIP) recommendation that people 65 years and older and those who are moderately or severely immunocompromised receive a second dose of the 2024-2025 COVID-19 vaccine six months after their first dose.
Announced on October 23, 2024, these updated recommendations also allow for flexibility for additional doses (3 or more) for moderately or severely immunocompromised people in consultation with their healthcare provider, known by the CDC as shared clinical decision-making.
In a press release, CDC Director Dr. Mandy Cohen stated, "This vote allows people to make the best decisions possible to keep themselves and their loved ones safe from COVID-19. CDC will continue to educate the public on how and when to get updated vaccinations so they can risk less severe illness and do more of what they love."

While the Los Angeles County Department of Public Health (LACDPH) says locally acquired dengue is rare in LA County, this year's infections will differ from 2023.
Last year, only two cases of locally acquired dengue were identified in Long Beach and Pasadena.
As of October 24, 2024, LACDPH has confirmed 9 cases of locally acquired dengue. Before their symptoms, these San Gabriel Valley residents had no history of travel to places where dengue is common.
These people live in Baldwin Park (6), Panorama City (1), and El Monte (2).
Since residents of LA County travel year-round, cases can occur at any time of year.
Most dengue cases in LA County involve traveling to countries where dengue outbreaks occur, such as the Caribbean (Puerto Rico), Central and South America, Southeast Asia, and the Pacific islands.
This year, 123 travel-related dengue cases have been confirmed in LA County, compared with 75 cases reported in LA County in 2023.
To alert international travelers of this health risk, the U.S. CDC reissued a Global Travel Health Notice on October 15, 2024, regarding Dengue outbreaks in 27 countries.
Dengue is a vaccine-preventable disease. However, protective vaccines will be unavailable in the U.S. in 2024.
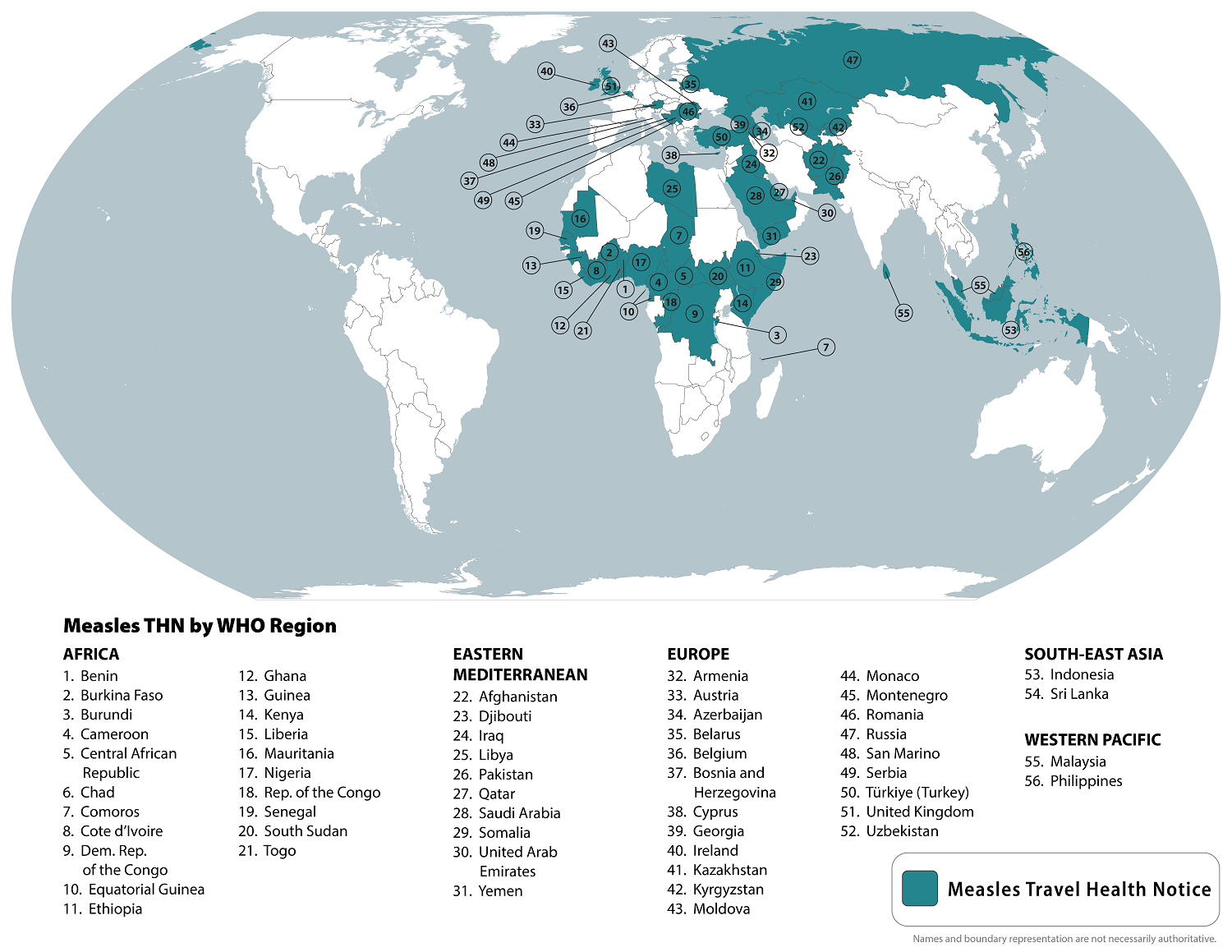
The World Health Organization recently reported that measles outbreaks have been reported in 103 countries in the last five years. According to an updated travel advisory, outbreaks have expanded in 2024.
The U.S. Centers for Disease Control and Prevention (CDC) reissued a Global Level 1 Travel Health Advisory on October 18, 2024, that lists 56 countries impacted by measles outbreaks.
The CDC says international travelers are at risk of measles if they have not been fully vaccinated at least two weeks before departure or have not had measles in the past and travel internationally.
Even in the United States, measles outbreaks have been reported this year in Chicago, Oregon, and New York City.
As of October 17, 2024, a total 269 measles cases were reported by 32 U.S. jurisdictions.
Measles vaccines are generally available at health clinics and pharmacies in the U.S.
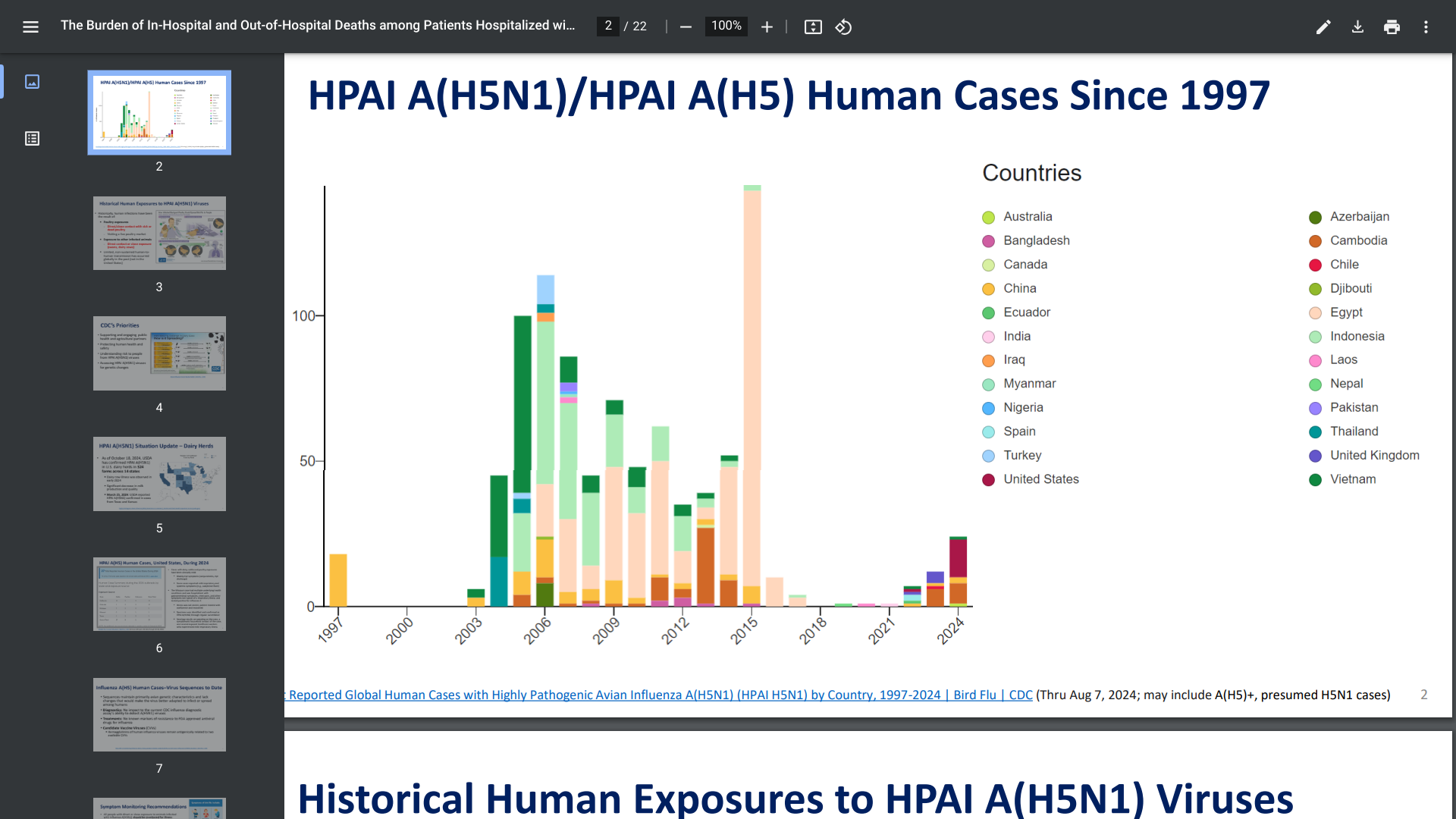
The U.S. CDC's Tom Shimabukuro, MD, MPH, MBA Influenza Division, presented an update on the recent Highly Pathogenic Avian Influenza (HAPI) A (H5N1) outbreak in dairy herds in the United States.
The USDA has confirmed HPAI A(H5N1) in U.S. dairy herds in 324 farms across 14 states, resulting in about 26 human cases.
On October 23, 2024, Dr. Shimabukuro concluded that the overall risk to the public for HPAI A(H5N1) remains low. However, exposed individuals (Michigan, Texas) should be monitored for (pink eye) symptoms after the first exposure and for 10 days and treated with approved influenza antivirals.
The CDC disclosed HPAI A(H5N1)/HPAI A(H5) human cases have been confirmed in numerous countries since 1997.
Furthermore, the CDC clarified that seasonal flu shots are not designed to protect people from 'Bird or Cow Flu' exposure.
If you plan to travel, you may need to be vaccinated against some diseases in other parts of the world. Some vaccines must be given well in advance to allow your body to develop immunity.
For some vaccines to become protective, several doses spread over several weeks or months.
According to the U.K. travel office on October 22, 2024, prospective international travelers should visit a G.P. or a private travel clinic at least 6 to 8 weeks before traveling bread in 2024.
In the U.K., the NHS routine immunization schedule lists vaccines that protect people against multiple diseases but does not cover all infectious diseases found overseas.
Furthermore, some countries require proof of vaccination (meningitis, polio, or yellow fever), which must be documented on an International Certificate of Vaccination or Prophylaxis (ICVP) before entering or leaving a country.
Even if an ICVP is not required, the U.K. recommends keeping a record of the vaccinations you have had with you, such as chikungunya, dengue, and measles.
In the United States, the CDC's travel advice website offers similar advice.

The U.S. CDC's Advisory Committee on Immunization Practices (ACIP) is scheduled to review scientific data and vote on vaccine recommendations on October 23-24, 2024.
Dr. Keipp Talbot, ACIP Chair, will lead this ACIP meeting from Atlanta, GA.
The final agenda includes presentations on chikungunya, HPV, influenza, pneumococcal, and RSV vaccines. These discussions are open to the public and available online via live webcast.
Several committee votes are planned during this meeting. The vote language shown at this link is considered draft language.
Furthermore, ACIP recommendations become official CDC policy once adopted by the CDC's Director.