Search API
Malaria vaccines have been in clinical development since the 1960s, with substantial progress over the past few years. In October 2021, the first of two malaria vaccines was approved to prevent Plasmodium falciparum malaria in children living in regions with moderate to high transmission.
However, recent studies have found maternal antibodies passed to infants can interfere with the response to the malaria vaccine.
The lower antibody titers in infants were attributed to either co-administration with routine vaccines included in the WHO Expanded Programme on Immunization, maternal anti-CSP antibodies, immune status regarding previous exposure, the infant's immature immune system, or a combination of these factors.
Published on October 23, 2024, this observational study conducted in six African countries, researchers concluded that interference between passive immunity and vaccine response is clinically significant and might affect the implementation of next-generation CSP-based vaccines for young infants and mothers and passive immunization with human monoclonal antibodies.
To validate this conclusion, additional clinical studies are being conducted.
As of late October 2024, malaria vaccines are offered in Africa, not the United States.
Bavarian Nordic A/S today announced the initiation of a clinical study of the MVA-BN® (JYNNEOS) mpox/smallpox vaccine in children 2 to 11 years of age, partially funded with $6.5 million from the Coalition for Epidemic Preparedness Innovations (CEPI).
The phase 2 study is currently enrolling children in the Democratic Republic of Congo, with plans to include sites in Uganda. Results from this study could support an extension of the current approval of MVA-BN to include young children.
Last month, the WHO prequalified MVA-BN for adolescents 12 to 17 years of age, adopting the recent approval from the European Medicines Agency (EMA) for this age group.
While this study represents the first investigation of MVA-BN as a mpox/smallpox vaccine for younger children, a recombinant version of MVA-BN (Mvabea®) was approved by EMA in 2020 as part of a prime-boost vaccine regimen or the prevention of disease caused by Zaire Ebolavirus.
Paul Chaplin, President and CEO of Bavarian Nordic, said in a press release, “Following the recent approval of MVA-BN for adolescents, we are pleased to initiate this study, which could provide additional data to extend the indication to include children. We thank CEPI and our partners in Africa for their support of this important work.”
From an access perspective, Bavarian Nordic announced an agreement with UNICEF on September 26, 2024, to supply 1 million doses of the MVA-BN® mpox vaccine for African countries impacted by the ongoing mpox outbreak.
As of late October 2024, there have been about 2,230 clade 2 mpox cases reported to the U.S. CDC this year.
The JYNNEOS vaccine is commercially available in the United States at various clinics and pharmacies.

Sandra Levy, senior editor of Drug Store News, conducted a digital conversation with Tim Ducharme, vice president of Immunization Growth and Strategy at CVS Health®.
This discussion, posted on YouTube on October 28, 2024, centered around the company’s vaccination business and its offerings for the respiratory virus season, and how it is working with patients to help them schedule any immunizations ahead of time.
Ducharme highlighted three areas of focus: appointment scheduling, vaccine availability, patient access issues and opportunities.
He also discusses how CVS is working to educate patients about the 2024-2025 respiratory season, which includes RSV and influenza. Various vaccines are available for these diseases.
Product delivery, available through the more than 9,000 CVS Pharmacy locations across the U.S., is a core component of the company’s digital offerings and efforts to meet consumers’ unique health and wellness needs.
The Iowa Department of Health and Human Services (IDHHS) today announced the death of an Iowa resident from Lassa fever, a rare, often fatal, viral hemorrhagic fever.
There have been eight travel-associated cases of Lassa fever in the U.S. in the past 55 years.
If confirmed, the Iowa case would be the ninth known occurrence of travelers returning to the U.S. since 1969.
This individual had recently returned from travel to West Africa, where ISHHS believes the person contracted the virus. About 5,000 Lassa fever-related deaths occur in West Africa each year.
Dr. Robert Kruse, State Medical Director of the IDHHS, stated in a press release on October 28, 2024, "I want to assure Iowans that the risk of transmission is incredibly low in our state. We continue investigating and monitoring this situation and are implementing the necessary public health protocols."
Lassa fever is carried by rodents and is transmitted to humans who may come in contact with the urine or feces of the infected rodents. Approximately 80% of Lassa fever patients have mild or no symptoms.
The U.S. Centers for Disease Control and Prevention posted a statement emphasizing that the overall risk to the public from this case is very low.
Lassa is included in the World Health Organization's R&D Blueprint of priority pathogens, for which there is an urgent need for accelerated research, vaccine development, and countermeasures.
As of October 2024, several Lassa fever vaccine candidates are conducting research, but the U.S. FDA has approved none.
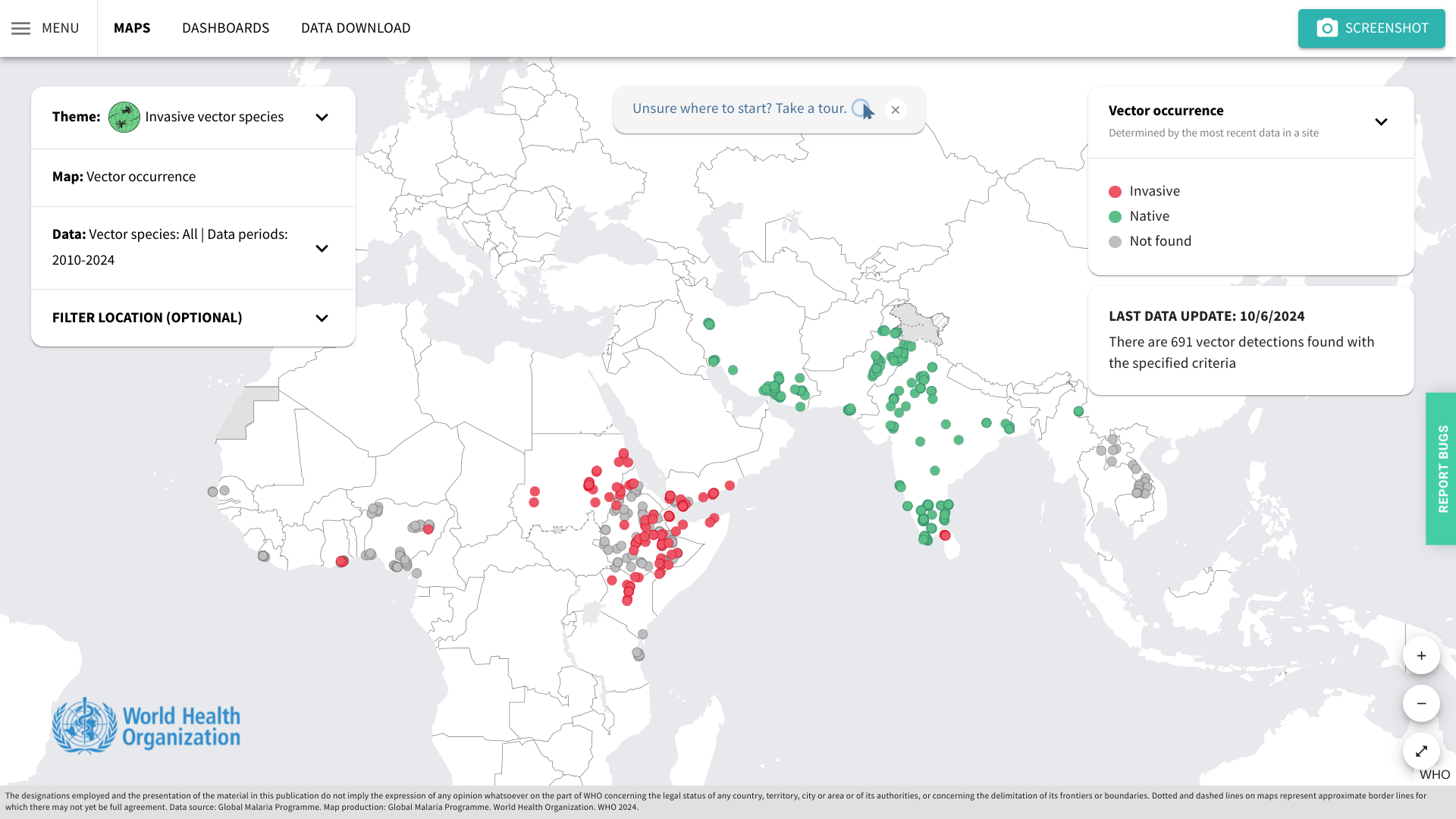
Although the risk for locally acquired malaria in the United States remains very low, its reemergence highlights the importance of vectorborne disease preparedness and response.
On October 24, 2024, the U.S. CDC published MMWR 73(42);946–949 confirmed ten local cases identified in Arkansas, Florida, Maryland, and Texas in 2023.
So far, in 2024, the CDC has confirmed 1,576 travel-related malaria cases, with New York City leading with 220. About 240 people with malaria are reported each year in NYC.
However, the CDC has not reported any local malaria cases this year.
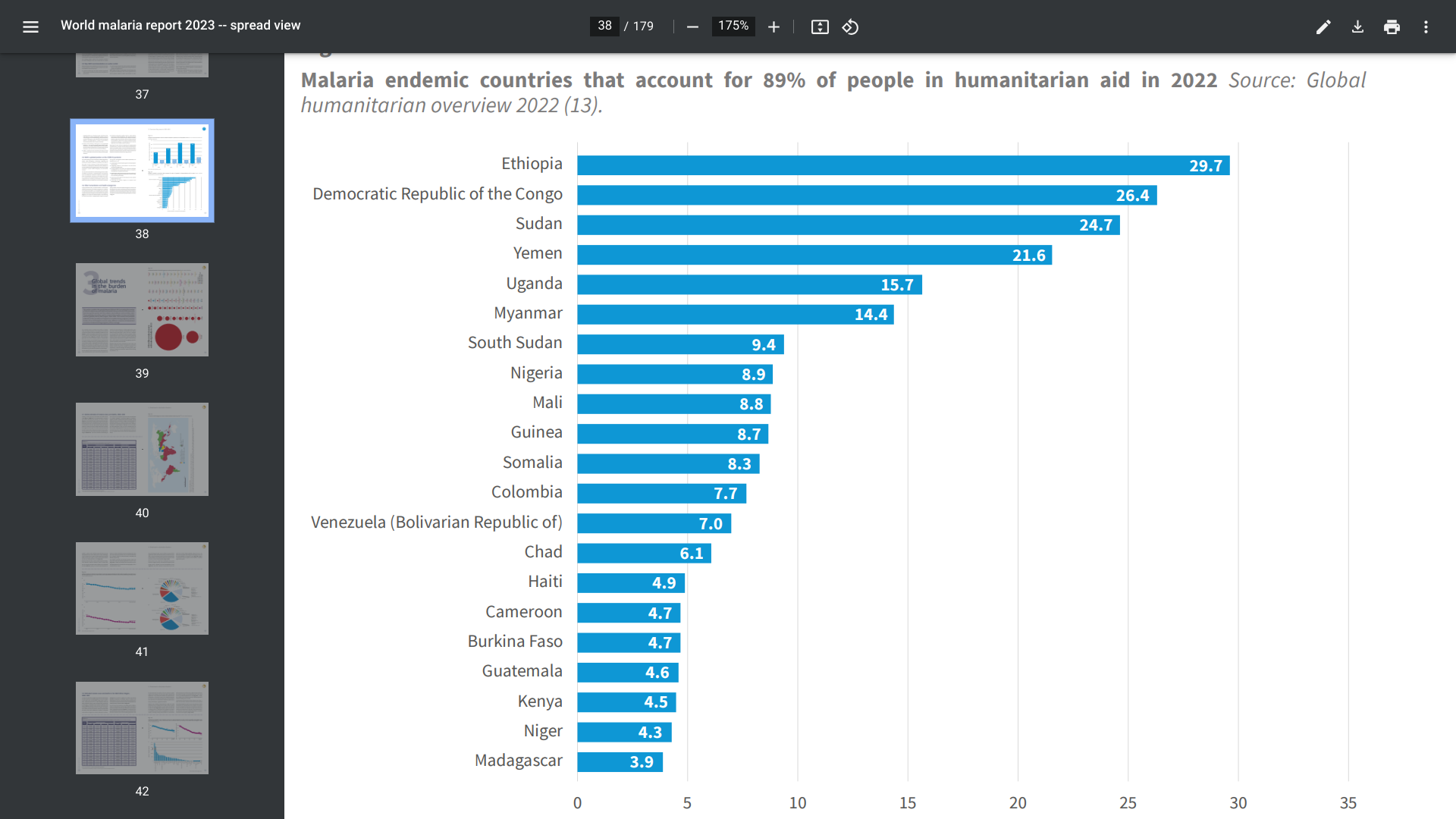
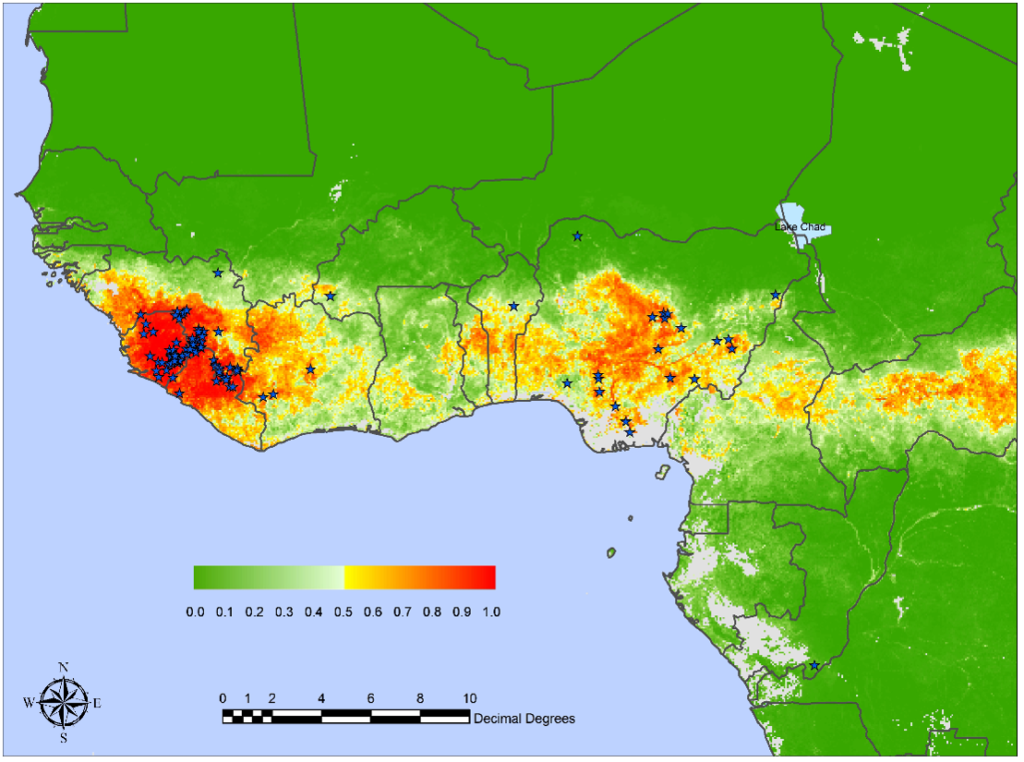
The WHO African Region shoulders the heaviest burden of malaria disease. When visiting malaria-endemic areas, the CDC recommends avoiding mosquito bites and bringing appropriate medications on your trip.
As of October 28, 2024, malaria vaccines are offered in Africa and Europe.
Vaxxas today announced that the U.S. National Institutes of Health (NIH) has granted the company a license to a next-generation vaccine antigen (DS2) designed for use in prophylactic vaccines against Respiratory Syncytial Virus (RSV).
DS2 has been found to prompt a more robust and durable immune response against RSV than the antigen used in globally approved vaccines (DS-Cav1).
There are three RSV vaccines currently approved for use in the U.S.
"Published preclinical results show the potential immunogenic advantages of this next-generation antigen as the basis for an RSV vaccine that could offer more robust and durable protection against the virus, compared to vaccines already on the market,” David L. Hoey, President and CEO of Vaxxas, in a press release on October 28, 2024.
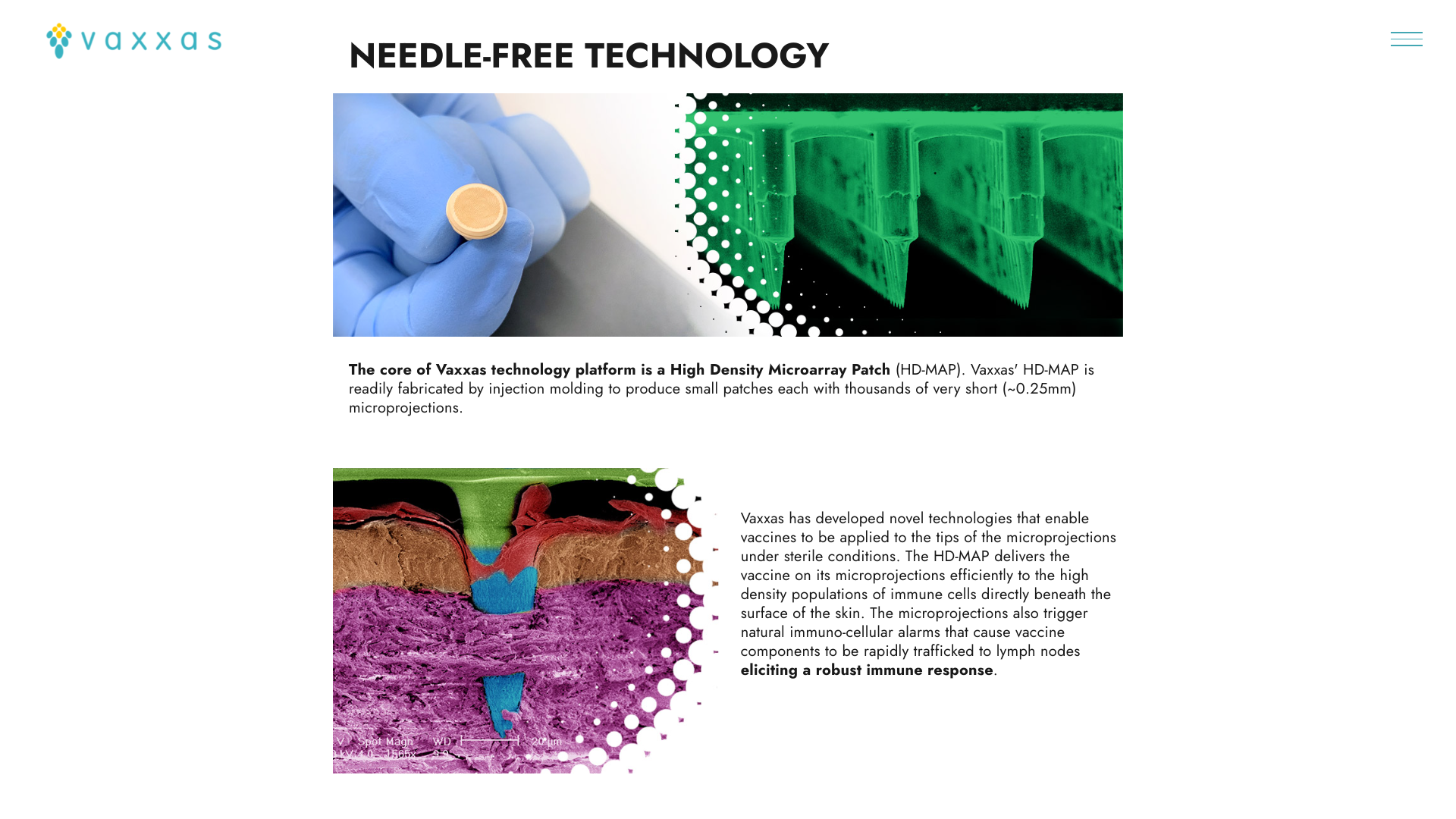
Furthermore, Vaxxas’ proprietary HD-MAP offers the potential for the first needle-free, room-temperature stable RSV vaccine.
The Vaxxas HD-MAP comprises thousands of microscopic projections molded into a small patch. Each microprojection is coated with a small dose of vaccine in a dried formulation. When applied to the skin using a proprietary applicator, the patch delivers the vaccine to the abundant immune cells that naturally reside immediately below the skin surface.
The company says, 'Ultimately, HD-MAPs could enable a future in which vaccine patches could be shipped directly to people, avoiding the delay, inconvenience, and safety challenges associated with traditional needle-and-syringe vaccine scheduling and administration.'