Search API
Novavax, Inc. today announced that the U.S. Food and Drug Administration (FDA) has cleared the Company to begin enrolling the planned Phase 3 clinical trial following the determination that Novavax satisfactorily addressed all clinical hold issues regarding its COVID-19-Influenza Combination and stand-alone influenza vaccine candidates.
"We thank the FDA for their partnership and thorough review of the additional information provided as part of our response package," said Robert Walker, MD, Chief Medical Officer, Novavax, in a November 11, 2024 press release.
"We plan to start our Phase 3 trial as soon as possible."
The Company did not estimate the availability of these innovative vaccine candidates.
Novavax is a global company advancing protein-based vaccines with its Matrix-M™ adjuvant, such as the R21/Matrix-M™ malaria vaccine currently available in Africa.

The World Health Organization (WHO) today published its 42nd situation report for the multi-country outbreak of mpox, which provides an update on the epidemiological situation of mpox in the WHO African Region and countries in the WHO Eastern Mediterranean Region.
The number of mpox cases in Africa is generally rising, driven mainly by cases reported from the Democratic Republic of the Congo (DRC).
As of November 11, 2024, the WHO confirmed Clade Ib monkeypox virus (MPXV) had been detected in six provinces in the DRC: South Kivu, North Kivu, Kinshasa, Kasai, Tshopo, and Tanganyika. Additionally, 11 other African countries have also reported clade Ib MPXV cases.
Since the last WHO situation report, three countries outside of Africa have confirmed travel-related cases of clade Ib MPXV,
For the first time, local transmission of clade Ib MPXV in the United Kingdom of Great Britain and Northern Ireland, where three (all) household members of the initial case (who had traveled to affected East African countries with clade Ib) were confirmed to have mpox.
Most impacted countries offer access to Bavarian Nordic's JYNNEOS® (MVA-BN®) vaccine to prevent mpox infections. The first round of mpox vaccination has concluded in the DRC, with around 51 500 people vaccinated across six provinces.
Furthermore, the clade 2 outbreak that began in May 2022 continues in numerous countries, including the United States.
Over the past few days, announcements have indicated more people may visit the Republic of El Salvador next year.
On November 1, 2024, Volaris El Salvador and Miami International Airport officials celebrated the launch of four weekly nonstop flights between San Salvador and Miami, Florida. In 2025, additional U.S. cities will also offer direct flights to this Central American destination.
On November 8, 2024, the U.S. Embassy in El Salvador reported significantly reducing gang-related activity and associated crime in the last two years. Recognizing these positive changes, the U.S. Department of State reduced its travel advisory to Level 2.
To keep visitors informed of local issues, the State Department recommends using major highways and roads and minimizing travel outside metropolitan areas when visiting El Salvador. It also recommends that visitors enroll in the Smart Traveler Enrollment Program to receive alerts and make locating you in an emergency easier.
From a health perspective, the U.S. CDC says dengue outbreaks are a year-round risk in many parts of the world, including El Salvador. As of November 2024, over 7,200 dengue cases were reported, an increase from the 5,788 cases confirmed last year.
Additionally, chikungunya, another mosquito-transmitted disease, has been confirmed in 47 people this year.
"For U.S. travelers with plans to visit El Salvador, it's essential to receive the hepatitis A and typhoid fever vaccines before visiting because you'll want to sample many of the excellent Salvadorian foods while on vacation. Be sure you're up-to-date on all routine vaccines, like hepatitis B, flu, measles (MMR), and tetanus," Jeri Beales, MSN, R.N., informed Vax-Before-Travel.
"Mosquitos are also a problem throughout El Salvador, and cases of dengue fever are on the rise this year. Unfortunately, no vaccine is available in the U.S. to protect people against dengue, so be sure to use an insect repellant with at least 20% DEET."
"But, the good news for U.S. travelers is you can now vaccinate (IXCHIQ®) against the chikungunya illness and travelers no longer need to take malaria medication while in El Salvador because it has been eradicated from all parts of the country," added Beales, who leads Destination Health Clinic. This Boston-area travel health provider specializes in health education and vaccination for international travelers.
The CDC suggests visiting your healthcare provider at least a month before your trip to El Salvador to acquire necessary vaccines such as chikungunya or typhoid. Visit the CDC's page for the latest Travel Health Information.

In recent years, mosquito-transmitted Japanese encephalitis virus (JEV) outbreaks have occurred in many parts of southern and eastern Asia.
According to health agencies, JEV has extended beyond its traditional boundaries to Indonesia, Papua New Guinea, and the Torres Strait and has been detected in Victoria, Australia, since 2022.
To help protect people from this disease, Victoria's Chief Health Officer announced on October 31, 2024, that more Victorians would have protection this mosquito season, with the Allan Labor Government expanding the eligibility of the free JEV vaccine program across the state.
This means people in Alpine, Macedon Ranges, Mansfield, and Mitchell, as well as local government areas, can access the JEV vaccine.
Minister for Health Mary-Anne Thomas said in a media release, "Summer provides mosquitos with an ideal breeding ground. In addition to getting vaccinated against JEV, Victorians in high-risk areas should take simple actions, like wearing loose-fitting clothes and using mosquito repellent."
This announcement means ValnevaSE's IXIARO® (JESPECT®) vaccine is now available to about seven million people in 24 regional local government areas in southeast Australia at a higher risk of exposure to the virus.
Furthermore, over 110 million international visitors are expected to visit Victoria in 2024.
IXIARO is the only JEV vaccine approved by the U.S. Food and Drug Administration. The U.S. Department of Defense has relied on IXIARO since 2010 to protect personnel deployed to JEV-endemic areas.
Valneva recently announced that IXIARO/JESPECT sales increased by 31% in the first nine months of 2024 compared to 2023.

The global shift from a 2-dose human papillomavirus (HPV) vaccine regimen to a 1-dose schedule began in 2022 to address supply shortages that have historically left individuals from low- and middle-income countries unprotected.
According to a Medical News in Brief published today by The JAMA Network, this transition accelerated on October 4, 2024, when the World Health Organization (WHO) prequalified Cecolin®. This HPV vaccine, which protects people against HPV 16 and 18, is the fourth vaccine for single-dose use.
Estimates from the WHO indicate that the switch to a single-dose vaccine schedule resulted in an additional six million girls being vaccinated against HPV globally in 2023.
That year, 27% of girls aged 9 to 14 received a single dose of HPV vaccine, compared with 20% in 2022.
As of September, 57 countries, up from 37 last year, had adopted the new vaccination plan. However, the U.S. CDC recommends either a 2- or 4-dose schedule.
Currently, there are six HPV vaccines in use, and various candidates are conducting clinical studies.

The World Health Organization (WHO) recommends administering a single typhoid conjugate vaccine (TCV) dose to children in high-burden countries. To better determine the TCV's efficacy, researchers extended the follow-up of the TyVAC clinical trial to assess protection five years after vaccination.
Published by The Lancet (Volume 404, Issue 10461) on October 12, 2024, these researchers conducted a cluster randomized controlled trial (ISRCTN11643110) in Dhaka, Bangladesh, between 2018 and 2021.
This study identified a decline in the protection conferred by a single-dose TCV 3–5 years after vaccination, with the greatest decline in protection and immune responses observed in children vaccinated at younger ages.
These researchers suggest that a booster dose of TCV around school entry age might be needed for children vaccinated when younger than two years old to sustain protection against typhoid fever during the school years when the risk is the highest.
This recommendation is essential as about nine million typhoid cases and 93,300 related fatalities are reported annually worldwide. Typhoid fever is a life-threatening infection caused by the bacterium Salmonella Typhi. It is usually spread through contaminated food or water.
The WHO currently recommends four different TCVs. These vaccines have been established as safe, well-tolerated, and effective.
The WHO writes that travelers to destinations with a high risk of typhoid fever be offered a typhoid vaccination. TVCs are available at travel clinics and pharmacies in the U.S.
The Bill & Melinda Gates Foundation funded this study.

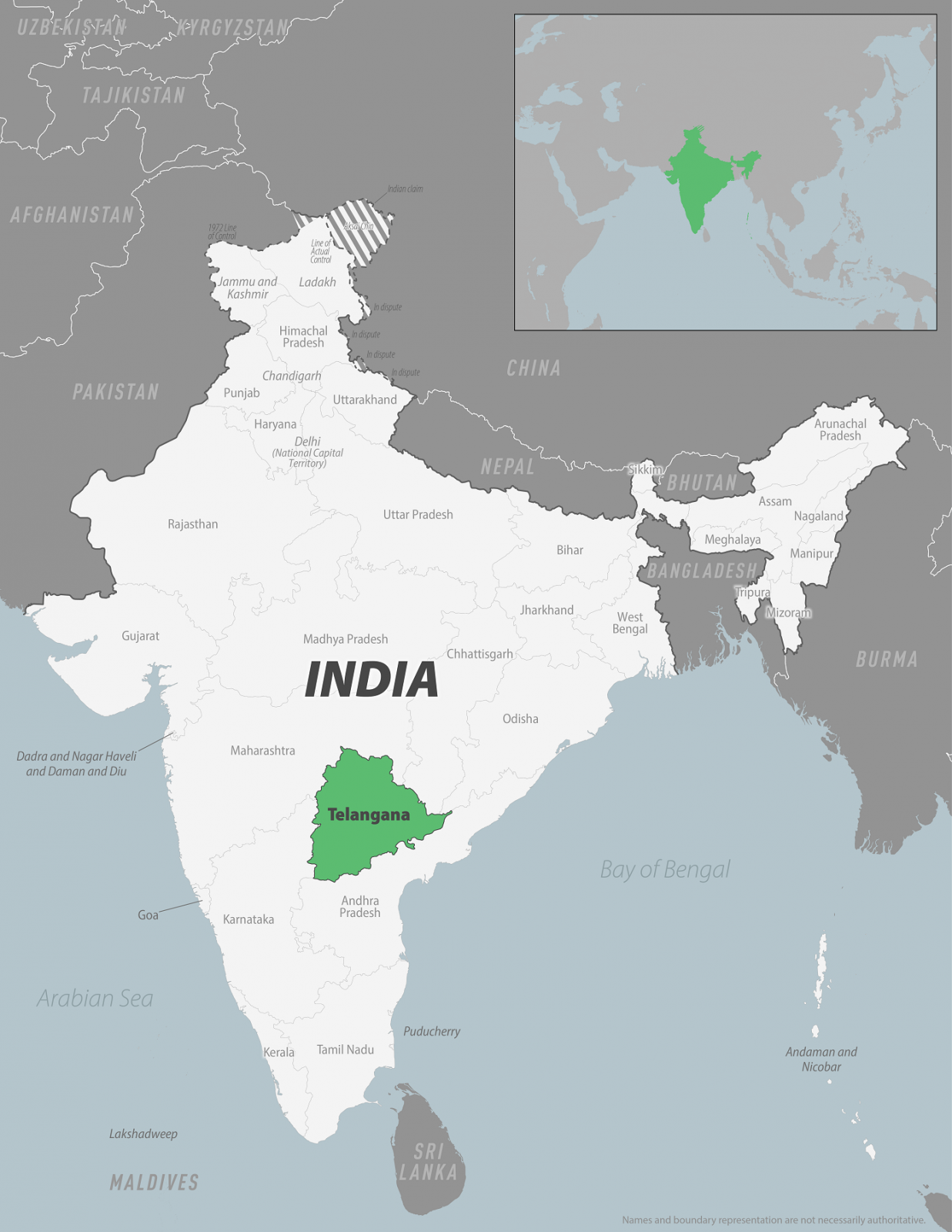
The U.S. Centers for Disease Control and Prevention (CDC) announced today it has identified a higher-than-expected number of chikungunya cases among U.S. travelers returning from the state of Telangana, India.
On November 8, 2024, the CDC issued a Level 2—Practice Enhanced Precautions, Travel Health Advisory to alert travelers to India's ongoing chikungunya outbreak. In 2024, India reported 69,395 chikungunya cases.
Telangana is located in the south-central region of India, with a population of about 35 million. The USA is generally the largest source of foreign tourists arriving in India annually.
As of early November 2024, the CDC reported that 153 people had been infected with chikungunya while traveling abroad. Last year, only 129 cases were confirmed.
People at risk for more severe disease include newborns infected around the time of birth, older adults, and people with medical conditions such as high blood pressure, diabetes, or heart disease. The CDC stated that if you are pregnant, reconsider travel to the state of Telangana, mainly if you are close to delivering your baby.
The CDC recommends vaccination against chikungunya for (most) adults traveling to a destination with a current outbreak.
In late 2023, the U.S. FDA approved Valneva SE's IXCHIQ®, the first vaccine to address chikungunya virus infections in adults. IXCHIQ is commercially available in the U.S. at most travel clinics and pharmacies.
The Global Polio Eradication Initiative (GPEI) confirmed that seven countries reported new polio cases involving wild poliovirus type 1 (WPV1) last week.
As of November 6, 2024, Pakistan reported 45 WPV1 cases this year. With 53 WPV1-positive environmental samples collected in September and October, Pakistan's outbreak may continue for months.
Five countries in Africa reported more vaccine-derived poliovirus cases, including Senegal, which had its first circulating vaccine-derived poliovirus type 2 (cVDPV2) case for the year. The other four are the Democratic Republic of the Congo, Ethiopia, Niger, and Nigeria.
In the Middle East, Yemen reported two cVDPV2 cases with paralysis onset in September.
The U.S. CDC's travel health advisory continues to identify 37 countries at risk for poliovirus outbreaks.
The CDC says that adults who previously completed the full, routine polio vaccine series may receive a single, lifetime booster dose of polio vaccine before traveling to any destination listed. Pre-departure polio vaccination services are offered at travel clinics and pharmacies in the U.S.


