Search API
The recently released World Health Organization (WHO) 2024 Global Tuberculosis Report revealed tuberculosis remains a leading infectious disease globally.
Unfortunately, the WHO African Region continues to be significantly impacted by tuberculosis (TB) outbreaks.
For example, Nigeria and the Democratic Republic of Congo (DRC) are among the eight countries that account for over 60% of the people estimated to have developed TB in 2023.
Nigeria has 4.6% of the global new cases, while the DRC has 3.1%.
When visiting Nigeria or the DRC, the U.S. CDC's Travel Health Notices do not recommend TB vaccination.
The only vaccine use to prevent TB is about 100 years.
Today, over ten versions of the Bacillus Calmette-Guérin (BCG) vaccine are used worldwide.
Over the decades, BCG vaccination has been found effective for children but less so for adults. The good news is that various TB vaccine candidates are in late-stage development, with authorizations expected soon.

The Texas Department of State Health Services (DSHS) reports an increase in pertussis cases in 2024, with about triple the number of cases reported this year compared with the same period in 2023.
Texas has confirmed 772 pertussis cases in 2024, compared to 264 last year.
This DSHS announcement on November 12, 2024, follows a national trend, which has seen a more than fivefold increase in 2024 cases based on preliminary data posted by the U.S. CDC.
As of week #44, reported on November 2, 2024, CDC data show that more than five times (22,240) as many pertussis cases were reported compared to (4,209) at the same time in 2023.
According to a state-based analysis, Pennsylvania reported about 10% (2,462) of all pertussis cases in 2024.
Pertussis is a highly contagious illness caused by the bacteria Bordetella pertussis and is vaccine-preventable, says the CDC.
Early symptoms are very similar to the common cold. People may develop paroxysms (coughing fits) one to two weeks after the first symptoms start. The cough generally gets worse and becomes more common as the illness continues. It can cause people to vomit or make a “whoop” sound when breathing in.
DSHS advises clinicians to follow the recommendations below and report any cases to their local health department within one workday.

Novavax, Inc. today announced its financial results and operational highlights for the third quarter ended September 30, 2024. A few highlights are inserted below:
For the third quarter of 2024, total revenue was $85 million, compared to $187 million in the same period in 2023. And ended the quarter with $1 billion in cash and receivables.
Advanced preparation for Sanofi to assume lead commercial responsibility of Nuvaxovid™ COVID-19 vaccine for the 2025-2026 vaccination season in the U.S., Europe, and select major markets not currently subject to Novavax Advanced Purchase Agreements or existing partnership agreements.
Entered the U.S. market with an improved Nuvaxovid presentation and broader access, available in pre-filled syringes in over 30,000 locations across major pharmacy retailers and regional grocers.
John C. Jacobs, President and Chief Executive Officer of Novavax, commented in a press release on November 12, 2024, “In addition to progress on our other value drivers, this past quarter, we made significant progress defining our R&D strategy as we look to expand beyond COVID-19 and influenza."
"We intend to develop our early-stage pipeline with a disciplined approach, as we focus on areas where our technology can have a positive impact on public health and generate value.”
An example is the R21/Matrix-M™ vaccine that includes Novavax's proprietary saponin-based Matrix-M adjuvant. This malaria vaccine is manufactured by the Serum Institute of India Private Ltd and is available in various African countries in 2024.

ImmunityBio, Inc. today announced its financial results for the third quarter ended September 30, 2024. The company achieved a net product revenue of approximately $6 million during the three months ended September 30, 2024, surpassing net product revenue of $1 million in the prior quarter and analyst estimates.
ANKTIVA® (N-803), U.S. FDA-approved and commercially available since May 2024, is now widely accessible to patients through commercial and government insurance programs (VA, DoD, Medicare). ImmunityBio has secured coverage for over 200 million medical lives through medical reimbursement policies.
ANKTIVA is a cytokine interleukin-15 (IL-15) that plays a crucial role in the immune system by affecting the development, maintenance, and function of key immune cells—NK and CD8+ killer T cells—that are involved in killing cancer cells.
By activating NK cells, ANKTIVA overcomes the tumor escape phase of clones resistant to T cells and restores memory T cell activity, resulting in a prolonged duration of complete response.
“The U.S. launch of ANKTIVA for non-muscle-invasive bladder cancer (NMIBC) CIS continues to gain momentum, and we are pleased to see the clinical impact for patients,” said Richard Adcock, President and CEO of ImmunityBio, in a press release on November 12, 2024.
“The Centers for Medicare and Medicaid Services have issued our permanent J-code, effective January 1, 2025."
"Our submission of ANKTIVA for NMIBC CIS to the U.K.'s MHRA for potential approval demonstrates our plans for global expansion. Further, we anticipate an EU submission this quarter.”

Arcturus Therapeutics Holdings Inc. today announced that the U.S. Food and Drug Administration (FDA) has issued a “Study Can Proceed” notification for the Company’s Investigational New Drug application, ARCT-2304, a self-amplifying mRNA (sa-mRNA) vaccine candidate for active immunization to prevent pandemic influenza disease caused by H5N1 virus.
The sa-mRNA vaccine candidate is designed to make many copies of mRNA within the host cell after intramuscular injection to enhance the expression of haemagglutinin and neuraminidase antigens, thereby enabling lower doses than conventional mRNA vaccines.
“Arcturus is actively engaged with the U.S. government to prepare for the next pandemic, and clearance to proceed into the clinic with our STARR® self-amplifying mRNA technology is a key step in this important process,” said Joseph Payne, President & CEO of Arcturus Therapeutics, in a press release on November 11, 2024.
“The Phase 1 clinical trial is designed to evaluate the safety, reactogenicity, and immunogenicity of ARCT-2304 as a potential vaccine to protect against the highly pathogenic H5N1 avian influenza.”
The clinical study is funded by the U.S. Biomedical Advanced Research and Development Authority.
The U.S. and European vaccine agencies have previously approved avian and pandemic influenza vaccines and have recently awarded funding grants for (bird flu) vaccine candidates.

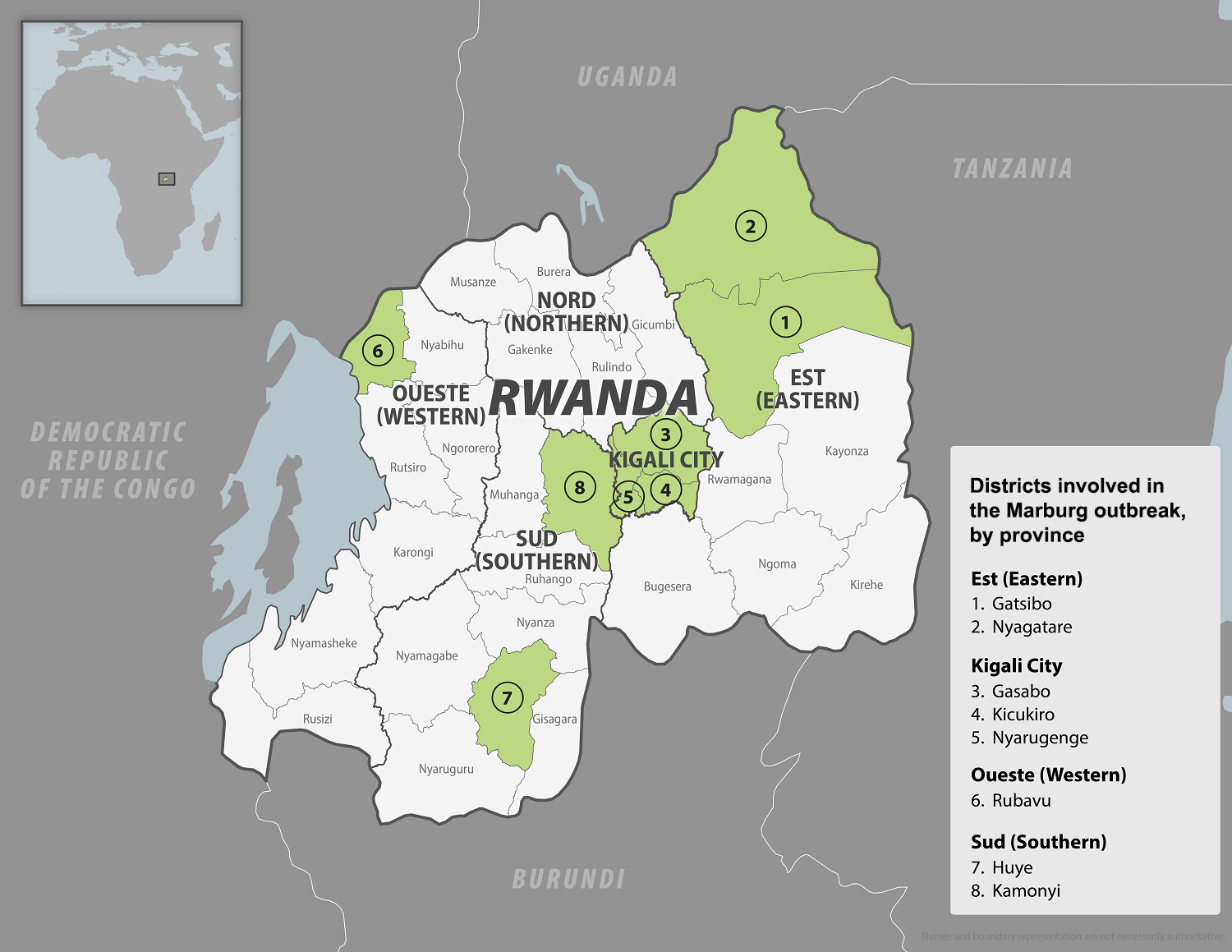
The WHO Africa recently announced that the Republic of Rwanda had discharged the last Marburg virus disease (MVD) patient, kicking off the mandatory 42-day countdown to declare the outbreak's end.
As of November 9, 2024, a total of 66 MVD cases and 15 deaths have been recorded during the outbreak, which was declared on September 27, 2024. Health workers, who constitute almost 80% of the cases, primarily became infected while providing emergency care to their colleagues and patients.
"This outbreak demonstrates that with the best available treatment, recovery is possible, and contributions to science can be made," said Dr Sabin Nsanzimana, Rwanda's Minister of Health, in a press release.
The World Health Organization published the Marburg vaccine development landscape on February 13, 2023. As of November 2024, no approved MVD vaccines exist.
Marburg is a highly virulent virus with a fatality ratio of up to 88%, and it was initially detected in Germany in 1967 following a lab incident. The virus belongs to the same family as the Ebola virus. Illness begins abruptly with high fever, severe headache, and malaise, and many patients develop severe hemorrhagic symptoms within seven days.
Currently, the U.S. CDC's Level 3—Reconsider Nonessential Travel Advisory remains active. The CDC recommends reconsidering nonessential travel to Rwanda, which is experiencing an outbreak of Marburg.