Search API
The Texas Department of State Health Services today is reporting an expansion of the measles outbreak in Texas.
As of February 18, 2025, the South Plains region has confirmed that 58 cases have been identified with symptom onset within the last three weeks. Fifty-four of these measles patients are unvaccinated or have unknown vaccination status.
Due to the highly contagious nature of this disease, additional outbreaks are likely to occur in Gaines County, Lubbock, Lynn, Terry, and Yoakum countries in western Texas.
DSHS released a media release stating it 'is working with South Plains Public Health District and Lubbock Public Health to investigate the outbreak.'
Just a few miles west of this Texas outbreak, the New Mexico Department of Health reported three residents tested positive for measles in February 2025.
Earlier in 2025, two measles cases were reported in the greater Houston area.
In 2024, the leading measles outbreaks in the U.S. were reported in Minnesota (70) and Illinois (67).
From a prevention perspective, the MMR vaccine has been found to be very effective against measles outbreaks. This vaccine is generally available at clinics and pharmacies throughout the United States.

Without an approved Lyme disease vaccine available, many people who enjoy the outdoors have concerns about planning hikes for Spring 2025. According to the World Health Organization, Lyme disease cases are common and expanding in Europe, the United Kingdom, and the United States.
But there is hope on the horizon.
Vaneva SE announced on February 18, 2025, that the first data readout for the Lyme disease vaccine candidate (VLA15) phase 3 is expected by the end of 2025.
This indicates that regulatory agencies may consider authorization in 2026. Valneva'shler, Valneva's Chief Financial Officer, commented in a press release, "Once again, we successfully delivered double-digit sales growth ...We made significant clinical and regulatory progress last year, setting the stage for several important catalysts to drive value in 2025, most notably with the first Phase 3 study results for our lead Lyme disease vaccine candidate, VLA15."
VLA15 is a multivalent recombinant protein vacBorrelia'sting Borrelia's outer surface protein A (OspA). It is designed for protective, active immunization against most human pathogenic Borrelia species. OspA is one of the most dominant surface proteins expressed by the bacteria when present in a tick bite.
A study published in late 2024 determined that 50% of adult blacklegged ticks carry the bacteria that causes Lyme disease, while up to 25% of the younger (nymph) blacklegged ticks carry the bacteria.
Until a preventive vaccine becomes commercially available, avoiding tick bites is the best way to prevent Lyme disease.

CSL and Arcturus Therapeutics recently announced that the European Commission (EC) has granted marketing authorization for KOSTAIVE ®, a self-amplifying mRNA COVID-19 vaccine, for individuals 18 and older.
KOSTAIVE (ARCT-154)is the first sa-mRNA COVID-19 vaccine to receive approval from the EC.
"KOSTAIVE and sa-mRNA technology signify a major advancement in vaccine innovation, providing the potential for broader and more enduring protection," said Joseph Payne, CEO of Arcturus, in a press release on February 14, 2025.
"This approval highlights the clinical promise of KOSTAIVE and its ability to protect against the ever-changing COVID-19 virus."
Unlike standard mRNA vaccines, self-amplifying mRNA vaccines instruct the body to make more mRNA and protein to boost the immune response.
KOSTAIVE is currently marketed in Japan against COVID-19.

GSK plc recently announced its MenABCWY vaccine PENMENVY could simplify meningococcal vaccination delivery and help protect more U.S. adolescents against these five common disease-causing serogroups – A, B, C, W, and Y, which commonly cause invasive meningococcal disease (IMD)/
To help realize that goal, the U.S. Food and Drug Administration has approved PENMENVY for use in individuals aged 10 through 25 years.
The vaccine combines the antigenic components of GSK's meningococcal vaccines, BEXSERO (Meningococcal Group B Vaccine) and MENVEO (Meningococcal [Groups A, C, Y, and W-135] Oligosaccharide Diphtheria CRM197 Conjugate Vaccine).
Tony Wood, GSK's chief scientific officer, said in a February 14, 2025 press release, "We are excited about the opportunities ahead to help improve meningococcal vaccination coverage in the United States, especially for IMD caused by serogroup B."
"Building on our global leadership in meningococcal vaccination and our longstanding commitment to addressing unmet needs in disease prevention, we aim to help protect more teens and young adults at a life stage when they are at an increased risk."
A positive vote on PENMENVY's use is expected at the U.S. CDC's Advisory Committee on Immunization Practices meeting on February 26, 2025.

Zoetis recently announced it received a conditional license from the United States Department of Agriculture (USDA), Center for Veterinary Biologics, for its Avian Influenza Vaccine, H5N2 Subtype, Killed Virus for use in chickens, not people.
On February 14, 2025, the company wrote that the decision to vaccinate commercial poultry flocks against Highly Pathogenic Avian Influenza (HPAI) rests solely with national regulatory authorities in partnership with the poultry industry.
"When a new strain of HPAI was identified in the U.S. in early 2022, our scientists immediately began work to update our previous avian influenza vaccine," said Mahesh Kumar, Ph.D., senior vice president of global biologics research and development at Zoetis, in a press release.
"We first worked on HPAI vaccines in 2001-02 when outbreaks occurred in flocks in Southeast Asia."
"Our readiness with this most recent vaccine is another example of how we continue to live our purpose to nurture the world and humankind by advancing care for animals, ultimately providing solutions to global animal health challenges."
In 2016, the company received a conditional license for its H5N1 vaccine and a contract award for the USDA's National Veterinary Stockpile; this vaccine was first used by the U.S. Fish & Wildlife Service in 2023 to help protect California condors. Zoetis also holds a USDA license used to help protect endangered birds in New Zealand in 2024.
The first participants have been vaccinated in a Phase 1 clinical study of a multivalent vaccine candidate designed to prevent skin and soft tissue infections (SSTIs) caused by the bacterial pathogen Staphylococcus aureus (S. aureus).
S. aureus infections pose a significant global health challenge, causing an estimated 1 million deaths annually. Notably, 90% of all community-acquired S. aureus infections are SSTIs.
Furthermore, the World Health Organization has designated S. aureus a "high priority" pathogen, underscoring the urgency of developing innovative vaccine approaches and effective treatment strategies.
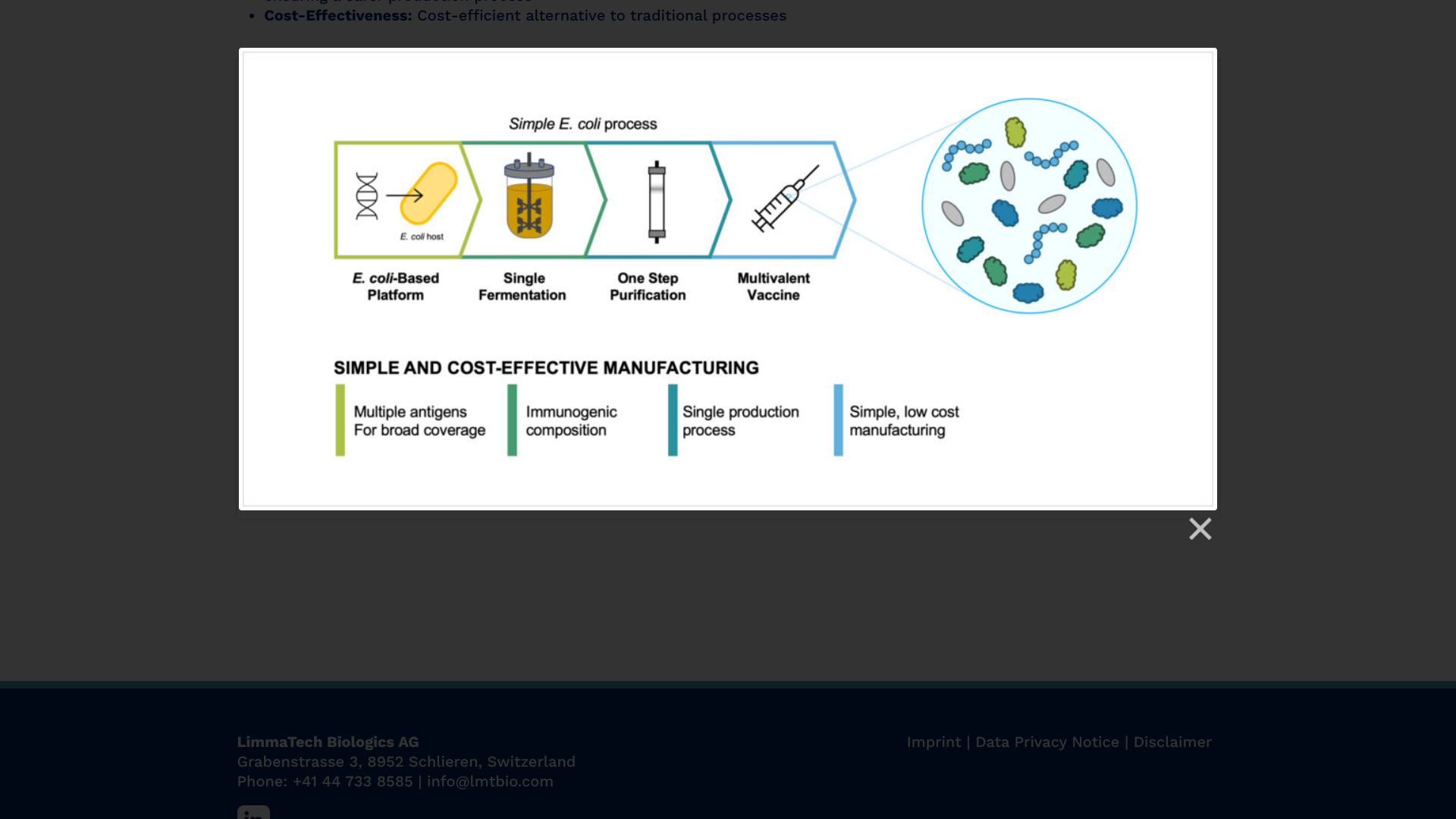
To address this essential health need, LimmaTech Biologics AG today announced that its LBT-SA7 vaccine candidate is expected to enroll 130 healthy adults aged 18-50 years, with initial results anticipated in the second half of 2025.
The company also announced the award of $6.5 million from the Combating Antibiotic-Resistant Bacteria Biopharmaceutical Accelerator (CARB-X) to advance the clinical development of LBT-SA7.
"Developing an S. aureus vaccine has long been a significant scientific challenge," explained Dr. Patricia Martin-Killias, Chief Operating Officer of LimmaTech, in a press release on February 17, 2025.
"We believe LBT-SA7 has the potential to provide a much-needed solution for those suffering from S. aureus infections. We are excited to launch the first-in-human clinical trial for LBT-SA7, bringing us closer to addressing an urgent global health challenge."
S. aureus is a Gram-positive bacterial pathogen that affects approximately 30% of the human population and causes a spectrum of infections, from SSTIs to severe conditions like pneumonia and bloodstream infections.
The company says traditional antibiotic treatments, both oral therapy and intravenous administration reserved for severe cases, have become increasingly less effective due to the rise of antibiotic resistance.
Federal funds from the U.S. Department of Health and Human Services partly fund CARB-X's funding for this project.
As the 2025 Daytona 500 NASCAR Cup Series starts-off today at Daytona International Speedway, over 150,000 people will be in attendance, many arriving by air.
The U.S. Transportation Security Administration (TSA) expects heavy travel volume from Daytona Beach International Airport (DAB) after the 500-mile race. The average daily passenger volume at DAB, around 1,000 passengers per day, will grow to an expected 2,200 on February 17, 2025.
"Planning is critical when traveling home after large events like this," said Brian Cahill, TSA Federal Security Director for DAB, in a press release. "Arriving at the airport with extra time and knowing what can and can't be packed in carry-on and checked bags will save you time and help keep things moving at checkpoints."
The good news for these travelers is that no health alerts have been issued for this area of central Florida.
The Volusia County Health Department has not issued any disease advisories this year.
However, travel-related and local diseases such as chikungunya, dengue, malaria, and Oropouche may impact race fans arriving from Miami-Dade County, located about 250 miles south along the east coast. Over the past few years, Florida's southeast coast has been a hot spot for mosquito-transmitted diseases.
As of February 2025, the U.S. CDC recommends two disease-prevention options: avoid mosquito bites and speak with a travel vaccine expert about U.S. FDA-approved immunization options. The CDC has issued Travel Health Advisories for numerous countries, but no alerts have been issued for the greater Miami area.