Search API
The Chicago Department of Public Health (CDPH) reconfirmed on June 27, 2023, it is investigating a recent increase in mpox cases among Chicago residents.
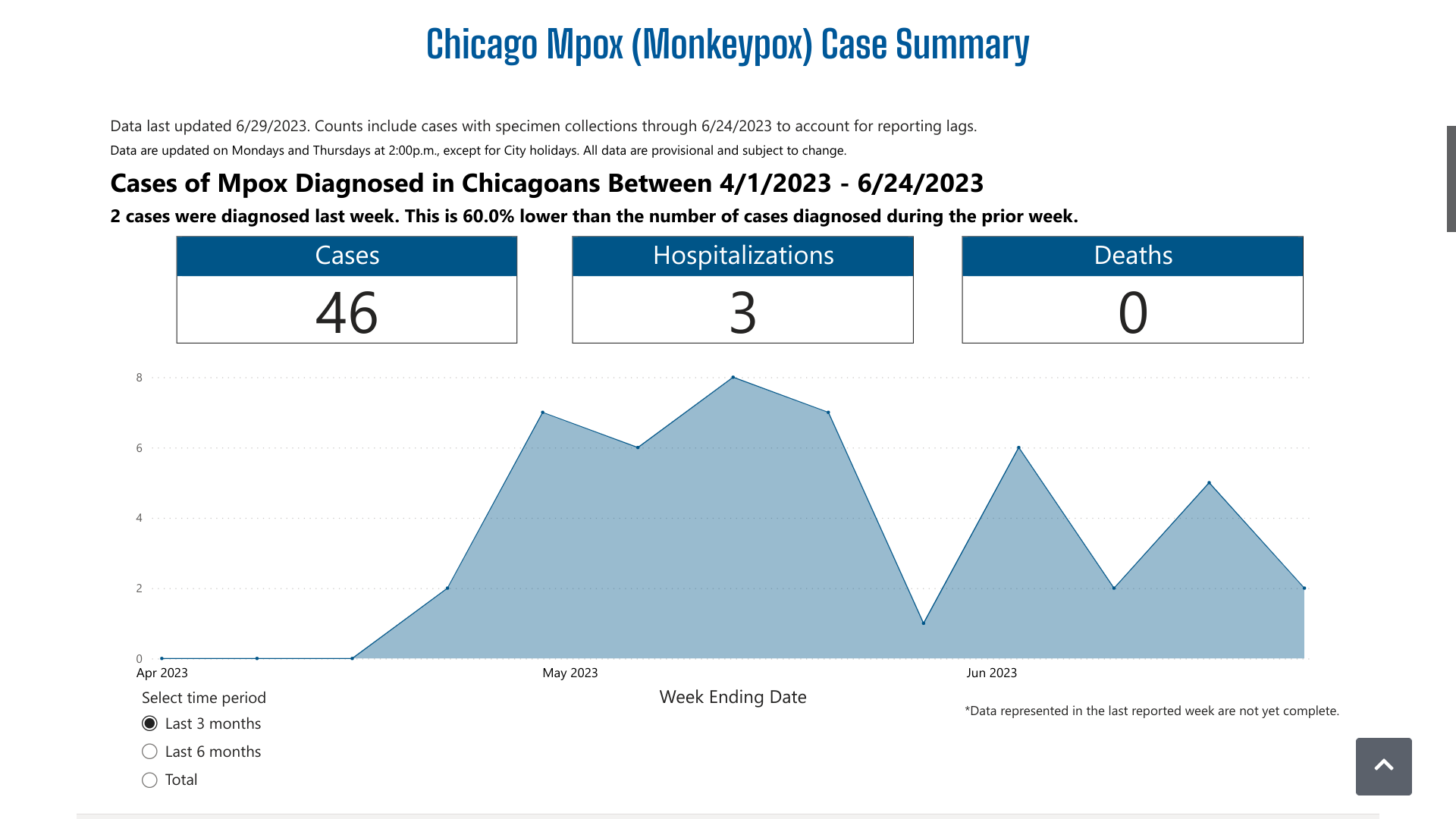
Between June 4 and June 22, 2023, there were six additional mpox cases reported.
The CDPH's dashboard indicates over the past three months, there have been 46 mpox cases and three related hospitalizations. And Chicago did not disclose the number of vaccine-breakthrough cases reported.
From a prevention perspective, 146 people were vaccinated in Chicago last week with the JYNNEOS® (MVA-BN) vaccine.
Since May 2022, 49,351 doses have been administered.
Chicagions with mpox questions can contact the HIV/STI Resource Hub at 844-482-4040 or the CDPH Call Center at 312-746-4835.
Throughout Illinois, including CDPH's data, 1,488 mpox cases have been reported during the global outbreak.
As of June 28, 2023, the U.S. CDC reported 30,531 mpox cases and 43 related fatalities since May 2022.
While Bavarian Nordic's vaccine has been the primary mpox vaccine offered in the U.S., a Cincinnati-based firm Blue Water Biotech, Inc., announced on June 28, 2023, preliminary preclinical data supporting the use of its norovirus shell and protrusion virus-like particle platform to develop a novel mpox vaccine candidate.
The World Health Organization (WHO) reported this week respiratory syncytial virus RSV activity was generally low worldwide except in Australia and a few countries in the Region of the Americas.
And as of June 26, 2023, RSV activity increased in a few tropical and temperate South American countries.
Furthermore, in the United Kingdom, the RSV detection rate among children under five years of age remained at a low level in June 2023.
In the U.S., Florida's RSV season is longer than the rest of the country, says the U.S. Centers for Disease Control and Prevention (CDC). For this reason, Florida is a good bellwether state for the forthcoming RSV season.
According to the Florida Department of Health, RSV activity was low in all five regions, with no outbreaks but an increased positivity rate as of June 24, 2023.
From a prevention perspective, the CDC approved RSV vaccines for people ages 60 and older, using shared Clinical Decision-Making.
This means these individuals may receive a single dose of the RSV vaccine based on discussions with their healthcare provider about whether RSV vaccination is right for them.

The U.S. Centers for Disease Control and Prevention (C.D.C.) today announced the current C.D.C. Director adopted the 2023-2024 Advisory Committee on Immunization Practices' (ACIP) recommendations on annual influenza (flu) vaccination for everyone six months and older in the U.S.
As of June 29, 2023, there are minor changes to the ACIP's flu shot recommendations, including, but not limited to, an acknowledgment of the updated flu vaccine composition for the 2023-2024 flu season.
And a change in the recommendations for vaccination of people with egg allergies.
Flu vaccination has many benefits. It has been shown to reduce the risk of getting sick with the flu and also to reduce the risk of more serious flu outcomes that can result in hospitalization or even death, says the CDC.
Rochelle P. Walensky, M.D., M.P.H.'s adoption of the ACIP recommendations makes them official C.D.C. policy. Providers should begin vaccinating patients according to C.D.C.'s recommended timing, which has not changed for the 2023-2023 influenza season in the U.S.
The C.D.C. says September and October are the best times for most people to get vaccinated.
Furthermore, flu vaccination in July and August is not recommended for most people, but there are several considerations for specific groups.
While influenza viruses are detected year-round, the exact timing and duration of flu seasons vary by country, says the World Health Organization (WHO). What happens in the Southern Hemisphere does not necessarily predict what will happen in the Northern Hemisphere, which includes the U.S.
The WHO recently published Influenza Update N° 448, which confirmed influenza detections remained low globally. Still, in the southern hemisphere, some countries reported variable changes in influenza detections in recent weeks, while detections in others seemed to have peaked as of June 26, 2023.
Additionally, Precision Vaccinations published an updated list of influenza vaccines and candidates conducting clinical trials.

The World Health Organization (WHO) today published an updated COVID-19 data dashboard indicating COVID-19 cases and related fatalities continue to decrease in most WHO Regions.
As of June 28, 2023, the African region reported a slight increase in COVID-19 fatalities but a 26% decrease in cases, while the other five WHO regions reported declines in both cases and deaths.
At the regional level, the number of newly reported 28-day cases decreased across the South East Asia Region (-78%), the Eastern Mediterranean Region (-71%), the Region of the Americas (-70%), the European Region (-46%), and the Western Pacific Region (-33%).
As of June 29, 2023, various COVID-19 vaccines remain available in most countries and may be required to enter some countries.

The MedAfrica Times recently reported that the Republic of Brazil donated 80 thousand vaccines against HPV and yellow fever to Cabo Verde.
The Cape Verdean Ministry of Health announced on June 27, 2023, 50 thousand doses of HPV and 30 thousand doses of yellow fever arrived in Praia.
"The yellow fever vaccine is used to prevent yellow fever, a disease caused by an arbovirus. It is recommended for prevention in endemic areas or for travelers", said this article.
The U.S. CDC recently wrote that no (health) notices are currently in effect for Cape Verde.
However, the CDC suggests prospective visitors to Cape Verde speak with a healthcare provider regarding travel vaccinations, such as yellow fever and medicines.
Located 900 miles south of the Canary Islands and 350 miles from the African mainland, Cape Verde's nine inhabited islands offer vacationers many relaxing options.
Cabo Verde has witnessed significant economic progress since 1990, driven in large part by the rapid development of tourism (25% of GDP).
And remember to pack essential health supplies in case of travel delays, says the CDC.

The U.S. Department of State recently published an updated Level 2 Travel Advisory for the Republic of Maldives, a nation located in the Indian Ocean.
On June 22, 2023, the State Department confirmed visitors to the Maldives should exercise increased caution due to civil unrest at tourist locations, transportation hubs, markets/shopping malls, and local government facilities.
And attacks may occur on remote islands, which could lengthen the response time of authorities.
Furthermore, U.S. citizens can obtain assistance from the U.S. Embassy Colombo in Sri Lanka.
If you travel to Maldives in 2023, the U.S. government suggests enrolling in the Smart Traveler Program to receive alerts during an emergency.
Moreover, getting to the Maldives is now easier.
The capital city of Malé recently announced the launch of Saudi Arabia's airline Flynas.
In 2022, Maldives welcomed over 1.6 million tourists.
From a health perspective, the U.S. CDC says no notices currently exist for Maldives.
However, the CDC encourages visitors to be current on several travel vaccinations, such as yellow fever, measles, and typhoid.