Search API
Anixa Biosciences, Inc. today announced that the U.S. Patent and Trademark Office (USPTO) issued a Notice of Allowance broadening protection of Anixa's novel ovarian cancer vaccine technology, which has been exclusively licensed from, and is being developed in partnership with The Cleveland Clinic.
This ovarian cancer vaccine targets the extracellular domain of anti-Müllerian hormone receptor 2 (AMHR2-ED), which is expressed in the ovaries but disappears as a woman reaches and advances through menopause.
However, AMHR2-ED is expressed again in the majority of ovarian cancers.
Dr. Amit Kumar, Chairman and CEO of Anixa, stated in a press release on July 27, 2023, "We are pleased to receive this notice of allowance from the USPTO, providing additional protection for our ovarian cancer vaccine technology, including a possible mRNA vaccine."
"Preclinical work to advance the vaccine is ongoing with support from the PREVENT Program at the National Cancer Institute, which supports preclinical innovative interventions and biomarkers for cancer prevention and interception."
The patent is titled "Ovarian Cancer Vaccines" and covers nucleic acid-based vaccine delivery. The late Dr. Vincent Tuohy of The Cleveland Clinic in Ohio is the lead inventor.
As of July 2023, the U.S. FDA has not approved an ovarian cancer vaccine.
Silvia Licciulli, Ph.D., wrote on June 7, 2023, the concept of using vaccination to eradicate cancer by instructing the immune system to eliminate malignant cells has been pursued for decades with little success.
To be successful, cancer vaccine approaches must overcome significant hurdles, including low immunogenicity and the immunosuppressive effects of the tumor microenvironment.

With several recent incidents reported in Irish media, the U.S. Embassy in Dublin reminded U.S. citizens on July 25, 2023, to exercise good personal security practices while traveling abroad.
For example, an older Buffalo, New York musician was abused last week during an unprovoked assault near Store Street Garda station.
The U.S. Embassy Dublin encourages all citizens to be aware of their surroundings, especially when traveling in unfamiliar places, crowded locations, empty streets, or at night in July 2023.
U.S. Embassy in Ireland can be contacted at +353 1 668-8777 or [email protected].
Furthermore, the U.S. Department of State reissued a Level 1: Exercise Normal Precautions travel advisory of Ireland on July 26, 2023.
The State Department says if you decide to travel to Ireland, visitors should participate in the Smart Traveler Enrollment Program to receive Alerts during an emergency.
From a health perspective, the U.S. CDC suggests speaking with a healthcare provider one month before visiting Ireland to ensure you are fully immunized against routine and travel diseases.

GSK plc today announced Shingrix®, a vaccine against herpes zoster (shingles), had achieved a U.S. cumulative immunization rate that grew from 30% at the end of 2022 and has now reached 32% at the end of Q1'23.
And sales increased in the second quarter of 2023, reaching 880 million pounds (about $1.1 billion).
However, U.S. sales declined 10% in the quarter, impacted by unfavorable wholesaler and distributor inventory movements plus lower non-retail demand partly offset by strong retail growth and pricing.
On October 20, 2017, the U.S. FDA authorized Shingrix.
Furthermore, Shingrix is now available in 33 countries, with recent additions in India and Japan.
Emma Walmsley, Chief Executive Officer, GSK said in a press release on July 26, 2023, "We have made a strong start to 2023, with excellent performance across Vaccines, Specialty and General Medicines."
"We are very focused on our upcoming launches, including our potential RSV older adult vaccine, and on continuing to strengthen our pipeline – both organically with several positive late-stage read-outs already this year, and through targeted business development."
In the U.S., the CDC says an estimated 1 million people get shingles yearly. If you've ever had chickenpox, you can get shingles. Even children can get shingles. Your risk of shingles increases as you get older.
GSK says people should not receive Shingrix if they are allergic to its ingredients or have had an allergic reaction to a previous dose of Shingrix.

MenFive®, the first conjugate vaccine to protect against the five predominant causes of meningococcal meningitis in Africa, has been prequalified by the World Health Organization (WHO).
Developed through a 13-year collaboration between Serum Institute of India Pvt. Ltd. (SIIPL) and PATH, MenFive® protects against meningococcal serogroups A, C, W, Y, and X and is designed to eliminate annual meningitis outbreaks and epidemics in the African meningitis belt.
It is also the only vaccine preventing meningitis caused by meningococcal group X, a pathogen increasingly implicated in African meningitis outbreaks.
Adar Poonawalla, CEO of Serum Institute of India, said in a media release on July 12, 2023, "As the first conjugate vaccine to safeguard against the five predominant causes of this deadly disease, MenFive® offers hope for a future free from annual outbreaks and epidemics in the African meningitis belt."
WHO prequalification ensures a vaccine meets strict international quality, safety, and efficacy standards, was supported by extensive clinical studies in The Gambia, India, and Mali that demonstrated a high level of safety and immunogenicity.
Importantly, prequalification allows MenFive® to be procured by United Nations agencies and Gavi, The Vaccine Alliance.
Meningitis is the inflammation of the tissues surrounding the brain and spinal cord. It is usually caused by infection.
Meningococcal meningitis is a bacterial infection that sets in rapidly and can kill within hours. It can cause severe brain damage and sepsis, leading to limb amputation, and is fatal if untreated in 50% of cases, says the WHO.
Anyone can contract meningococcal meningitis, but children under age five—especially infants—are likely to suffer the most severe effects.
In the United States, two types of meningococcal vaccines are licensed by the Food and Drug Administration: Meningococcal conjugate (MenACWY) vaccines and Serogroup B meningococcal (MenB) vaccines.

In the United States, community outbreaks of the respiratory syncytial virus (RSV) typically occur during late fall, winter, and early spring.
However, recent data indicates the state of Florida has been the location where RSV cases are initially reported each year.
As of July 22, 2023, the Florida Department of Health segmented reported RSV activity had been identified in a few counties, and the southeast area was in-season, but no community outbreaks.
The U.S. Centers for Disease Control and Prevention say RSV can be contagious for 3 to 8 days in an infected person and is spread by coughs or sneezes. And healthcare providers should consider RSV in patients with respiratory illness.
Infants and children with RSV infection may have rhinorrhea and decreased appetite before other symptoms appear. Additionally, some infants and people with weakened immune systems can spread RSV after not showing signs for up to one month.
As of July 18, 2023, laboratories reported to the CDC the total number of RSV tests performed and those tests that were positive.
From a prevention perspective, the U.S. FDA has recently approved RSV vaccines and a second antibody therapy for children.

Moderna, Inc., a biotechnology company pioneering messenger RNA (mRNA) therapeutics and vaccines, and Merck today announced the initiation of the pivotal Phase 3 randomized V940-001 clinical trial evaluating V940 (mRNA-4157), an investigational individualized neoantigen therapy (INT), in combination with KEYTRUDA, Merck's anti-PD-1 therapy, as an adjuvant treatment in patients with resected high-risk (Stage IIB-IV) melanoma.
INTs are designed to train and activate the immune system so that a patient can generate an antitumor response specific to their tumor mutation signature.
V940-001 is the first Phase 3 study of a planned comprehensive clinical development program initiated following the positive primary analysis of the Phase 2b KEYNOTE-942/mRNA-4157-P201 trial.
Global recruitment in V940-001 has begun, and the first patients are now enrolling in Australia.
"The initiation of the V940-001 Phase 3 trial is an exciting and important milestone for us as we work with our colleagues at Merck and the melanoma patient community to investigate how individualized neoantigen therapy may potentially transform the treatment of the most serious form of skin cancer," said Kyle Holen, M.D., Moderna's Senior Vice President and Head of Development, Therapeutics and Oncology, in a press release on July 26, 2023.
Melanoma, the most serious form of skin cancer, is characterized by the uncontrolled growth of pigment-producing cells.
Nearly 325,000 new cases were diagnosed worldwide in 2020.
In the U.S., skin cancer is one of the most common types of cancer diagnosed, and melanoma accounts for a large majority of skin cancer deaths. It is estimated there will be nearly 100,000 new cases of melanoma diagnosed and almost 8,000 deaths resulting from the disease in the U.S. in 2023.

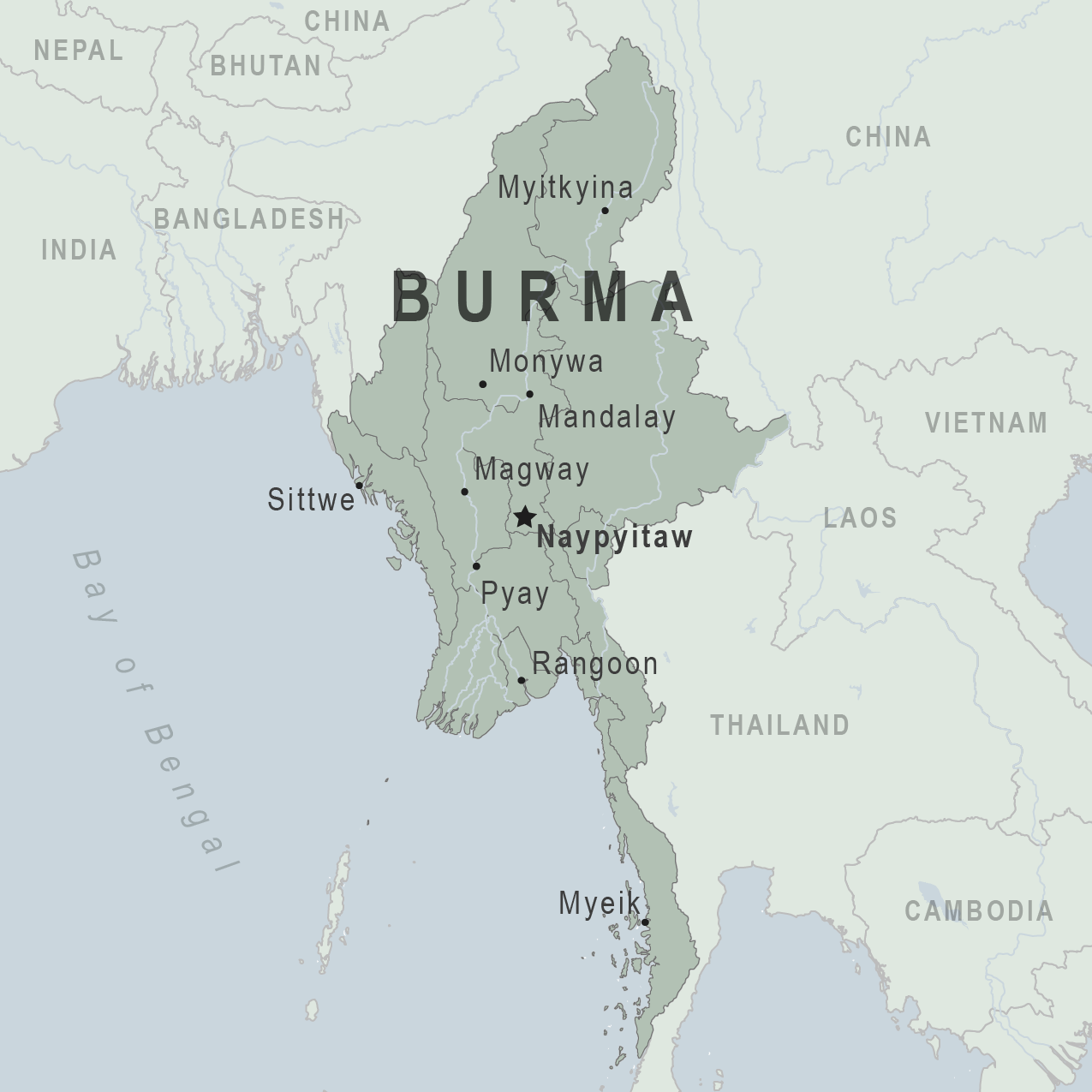
A high-level travel advisory was reissued today for the Republic of the Union of Myanmar (Burma), disclosing various reasons not to visit this Southeast Asian nation in 2023.
On July 24, 2023, the U.S. Department of State's Level 4 Travel Advisory highlighted civil unrest and the risk of wrongful detention of U.S. nationals by the military regime exists.
Additionally, the U.S. government has limited ability to provide emergency services in Myanmar as U.S. government employees must obtain special authorization to travel outside of the city of Yangon (Rangoon).
Furthermore, visitors should exercise increased caution due to wrongful detentions and areas with land mines and unexploded ordnance.
From a health perspective, the State Department confirmed limited and/or inadequate healthcare resources in Myanmar.
And recently, the U.S. CDC confirmed Dengue is a risk in many parts of Asia and the Pacific Islands, including Myanmar.
If you intend to visit Myanmar, the CDC suggests speaking with a healthcare provider about routine and travel vaccinations.
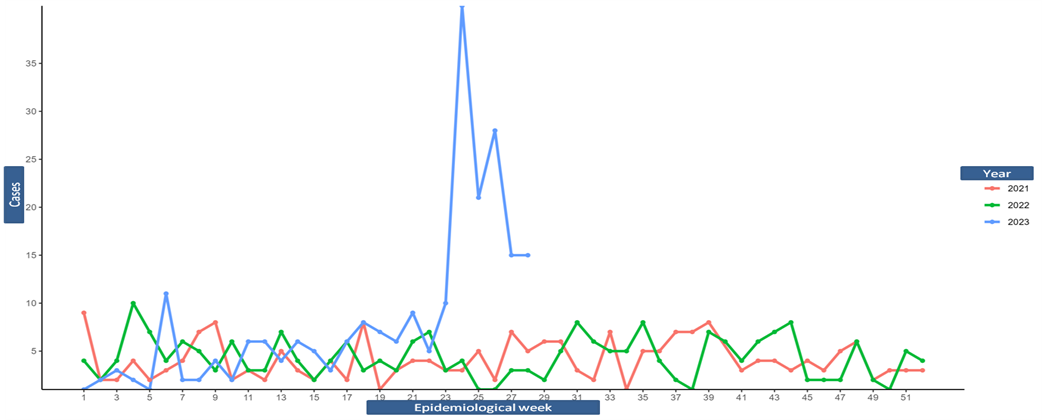
Over the past eighteen months, numerous reports of avian influenza infections in birds, mammals, and even people in 47 states have been reported.
Since January 2022, based on its genetic features, about 836 flocks, and over 58 million birds have been infected with the highly pathogenic avian influenza (HAPI) virus.
To date, more than 6,500 people in 52 jurisdictions have been monitored since 2022, and only one human case has been identified in the U.S.
However, the U.S. Animal and Plant Health Inspection Service posted some excellent 'bird flu' news on July 25, 2023.
There has not been a confirmed bird flu outbreak in the U.S. since May 18, 2023.
On July 7, 2023, the U.S. CDC published an updated Technical Report that confirmed the overall risk to human health associated with the ongoing outbreaks of highly pathogenic A(H5N1) viruses in wild birds and poultry has not changed and remains low at this time.
Should a bird flu outbreak occur in humans, the U.S. government has already invested in developing related vaccines.
The World Health Organization (WHO) today published an update Disease Outbreak News regarding the Republic of Peru's ongoing Guillain-Barré Syndrome (GBS) outbreak.
On July 25, 2023, the WHO reported 130 suspected cases of GBS between June 10 and July 15, 2023. Of these cases (94 cases) presented with upward progression of paralysis as a neurological manifestation.
Throughout 2023 (until July 15, 2023), a total of 231 cases have been confirmed.
To date, the potential cause of the unexpected GBS incidence remains under investigation.
Furthermore, the WHO and the U.S. CDC have not issued any recommendations to impose travel and/or trade restrictions in response to this GBS outbreak.
The Presidency of the Republic of Peru declared a national health emergency in early July 2023, due to the unusual increase and enhanced the implementation of public health responses.
In 2019, Peru reported an unprecedented outbreak of GBS that affected several regions of the country, with almost 700 reported cases.
From the clinical-epidemiological characteristics and the study of the identified agents, it was concluded that the outbreak was associated with the presence of the Campylobacter jejuni sequence type 2993 genotype.
The WHO says GBS is a rare neurological disorder of variable clinical severity, including fatal outcomes. It is the most common form of acute flaccid paralysis (AFP), a polio-like disease.
It is characterized by motor weakness, areflexia (absence of muscle reflexes), sensory abnormalities, and elevated protein levels in cerebrospinal fluid (cytoalbuminologic dissociation). Most often, an upper respiratory or gastrointestinal illness typically precedes GBS.
No known cure for GBS nor protective vaccines is available, says the WHO.