Search API
The World Health Organization (WHO) recently reported circulating vaccine-derived poliovirus type 2 (cVDPV2) cases in Africa.
The WHO confirmed on July 28, 2023, the United Republic of Tanzania reported the country's first cVDPV2 case and Kenya its first of 2023.
Tanzania's Ministry of Health notified the WHO the cVDPV2 virus was isolated from a case of acute flaccid paralysis (AFP) in the Rukwa region. Gene sequencing of the isolated virus has indicated close linkage with cVDPV2 currently circulating in South Kivu, Demographic Republic of the Congo.
According to the WHO-UNICEF estimates of national immunization coverage, the oral polio vaccine third dose (OPV3) and the inactivated polio vaccine first dose (IPV1) was 88% in Tanzania last year.
And on July 11, 2023, the WHO received an official report regarding detecting a cVDPV2 in two AFP cases and two asymptomatic healthy children community contacts in Kenya.
The genetic sequencing analyses showed that all four isolates are genetically linked to the cVDPV2 circulating in Banadir, Somalia.
Vaccine-derived poliovirus is a well-documented strain mutated from the strain originally contained in OPV.
OPV contains a live, weakened form of poliovirus that replicates in the intestine for a limited period, thereby developing immunity by building antibodies. On rare occasions, when replicating in the gastrointestinal tract, OPV strains genetically change and may spread in communities that are not fully vaccinated against polio, especially in areas with poor hygiene or overcrowding.
The lower the population's immunity, the longer this virus survives and the more genetic changes it undergoes.
In sporadic instances, the vaccine-derived virus can genetically change into a form that can cause paralysis, as does the wild poliovirus – this is what is known as a vaccine-derived poliovirus (VDPV).
The detection of VDPV in at least two different sources and at least two months apart that are genetically linked, showing evidence of transmission in the community is classified as cVDPV2.
In both countries, the WHO assesses the overall risk at the national level to be high due to the sub-optimal surveillance performance in some districts, sub-optimal vaccination coverage resulting in low population immunity, and the ongoing population movement across neighboring countries.
To alert international travelers, the U.S. CDC reissued its Level 2 - Practice Enhanced Precautions, Travel Health Advisory regarding the global polio outbreak on July 28, 2023.
The CDC says before any international travel, make sure you are up to date on your polio vaccines, and adults who previously completed the full, routine polio vaccine series may receive a single, lifetime booster dose of polio vaccine.
The nOPV2 vaccine is offered in Africa in July 2023. Approximately 670 million doses have been administered in more than 31 countries worldwide.
In the U.S., various polio vaccines are available at health clinics and community pharmacies.
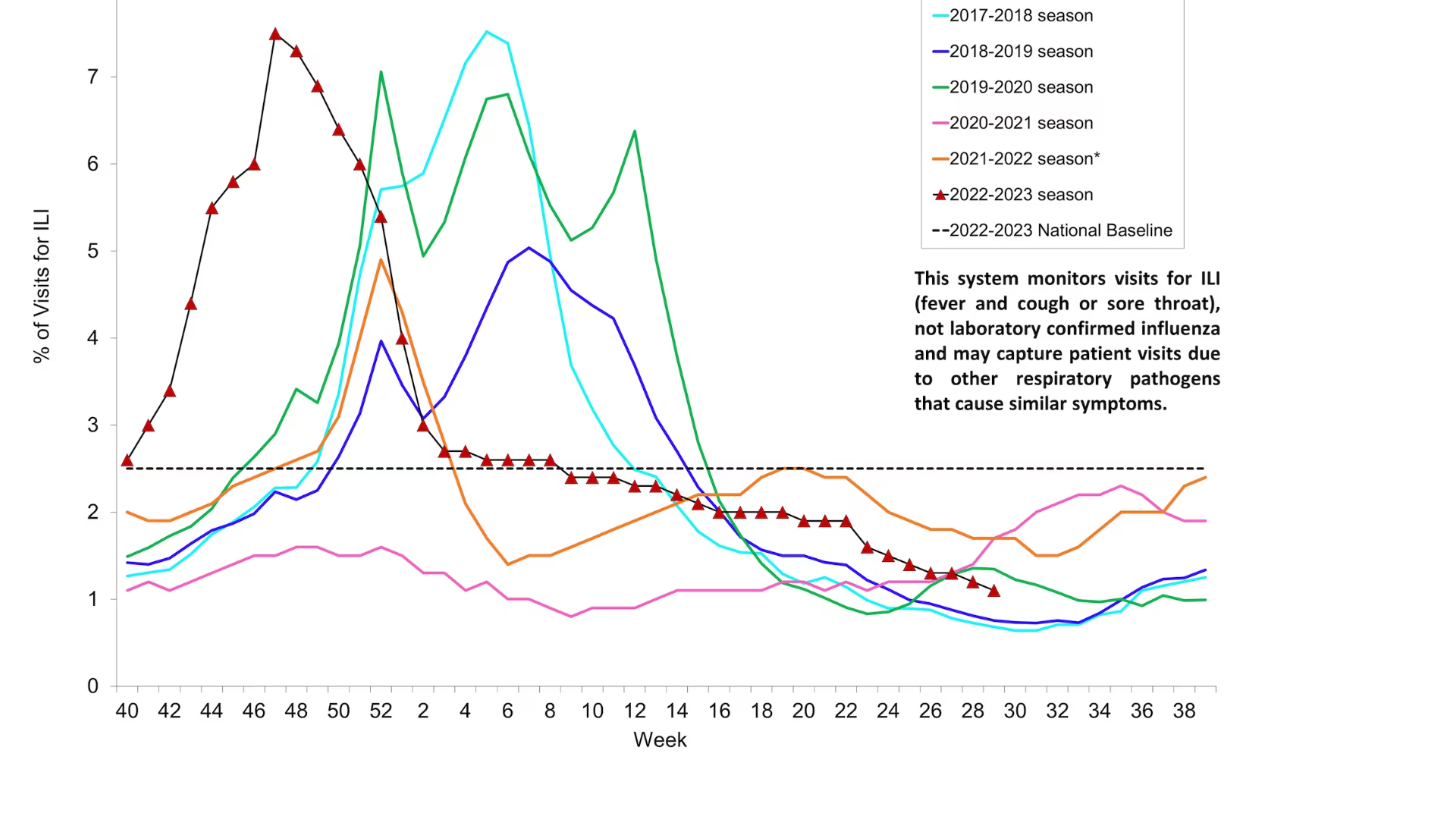
According to the World Health Organization (WHO)'s Influenza Season Update N° 450, influenza detections remained low globally.
As of July 24, 2023, in the temperate zones of the northern hemisphere, influenza activity was reported at low levels or below the seasonal threshold in most reporting countries.
And in the Caribbean and Central American countries, influenza activity remained low, with some countries reporting decreased activity.
Additionally, in Oceania, including Australia, influenza activity increased with the predominant influenza A viruses.
In the United States, the CDC reported for Week #29, ending July 22, 2023, seven additional death certificates listed influenza as an underlying or contributing cause of death.
Moreover, a total of 164 influenza-associated pediatric deaths occurred during the 2022-2023 season, according to the CDC on July 28, 2023.
Dallas County Health and Human Services (DCHHS) today reported two new human cases of West Nile Virus (WNV) infection in Dallas County for 2023.
The patients are two male residents of the 75228 and 75204 zip codes in the City of Dallas. Both patients were diagnosed with West Nile Neuroinvasive Disease (WNND.)
Due to privacy and confidentiality reasons, DCHHS does not disclose additional information about the patient.
As of July 28, 2023, there are four total human cases this year.
There were 42 cases of WNV in Texas and seven deaths in 2022. Over the last five years, Texas has had 485 cases and 65 deaths.
"Mosquito activity continues in our community, and we're now reporting two additional human cases of West Nile Virus. WNV is transmitted to humans by the bite of an infected mosquito, and people should be careful when going out outside to enjoy outdoor activities," said Dr. Philip Huang, DCHHS Director. "Please follow the 4 Ds to do everything you can to avoid mosquito bites," in a press release.
As of July 28, 2023, there were seven new mosquito sample pools: in Dallas, 75218, 75241, and 75243 (2 pools); Mesquite, 75150 (3 pools).
WNV is transmitted through the bite of infected mosquitoes, says the Texas Department of State Health Services (DSHS).
Most people exposed to the virus don't get sick, but about 20% develop symptoms like headache, fever, muscle and joint aches, nausea, and fatigue.
In a tiny proportion, less than 1%, the virus affects the nervous system, leading to the more severe WNND that can cause neck stiffness, disorientation, tremors, convulsions, paralysis, and even death.
"It's important for people to be aware that there are many diseases transmitted by mosquitoes found in Texas," said DSHS Commissioner Jennifer Shuford, MD, MPH, in a press release on July 11, 2023.
"Most of these diseases cause mild illness, but in rare instances, diseases like dengue or Zika can cause severe illness."
"We've even had a locally acquired malaria case in Texas this year, which underscores the importance of taking precautions to prevent mosquito bites."
As of July 2023, the U.S. FDA has not approved a WNV vaccine.

While various measles outbreaks continue globally, one country just reported some great news.
The Republic of South Africa's National Institute for Communicable Diseases (NICD) today announced in the past week, as of July 22, 2023, only one laboratory-confirmed measles case was detected from the Western Cape province.
And the Limpopo province, the year-long measles hot spot, is currently completing a mop-up vaccination campaign.
The outbreak is effectively over, said the NICD in a media release on July 28, 2023.
However, the percentage of samples testing positive increased from (0/19) in week 28 to (1/12.) of samples tested in week 29.
The NICD has tested 6,541 serum samples for measles since epidemiological week 40, 2022, of which 1,115 (17%) were confirmed positive.
According to the U.S. Centers for Disease Control and Prevention (CDC), the top ten measles outbreaks as of July 18, 2023, were led by India, with 67,592 cases.
To alert international travelers, the CDC published a global Watch-Level 1, Practice Usual Precautions notice on June 29, 2023, regarding measles outbreaks in Africa and other countries.
Measles cases worldwide increased by about 80% during 2022 compared with 2021.
And the CDC recently reported 18 measles cases in twelve jurisdictions in 2023. Last year, there were 121 measles cases in six U.S. jurisdictions.
According to recent news from the World Health Organization (WHO), respiratory syncytial virus (RSV) outbreaks were found in a few countries in the Region of the Americas.
The WHO's Influenza Update N° 449, published on July 7, 2023, RSV activity was increasing in a few temperate South American countries.
Separately, the U.S. Centers for Disease Control and Prevention (CDC) recently offered insights into when the U.S. could expect RSV to be detected in 2023.
The CDC's Morbidity and Mortality Weekly Report presented the seasonality of RSV in the U.S. from 2017–2023. The most recent RSV season onset occurred in June and peaked in November.
Across both prepandemic and pandemic years, RSV circulation in the U.S. began in Florida, then in the southeast, and later in the north and west regions.
The CDC says Florida's RSV season is longer than the rest of the U.S.
For this reason, the Florida Department of Health segmented reports into five RSV regions, each with its own RSV season. As of July 22, 2023, there were no RSV outbreaks in Florida.
From a prevention perspective, the U.S. FDA recently approved RSV vaccines and a second monoclonal antibody therapy for children.

The U.S. NIH’s Carl Dieffenbach, Ph.D., Director of the Division of AIDS at the National Institute of Allergy and Infectious Diseases, spoke with Louis Shackelford, MPH, on July 25, 2023, about HIV vaccine studies being discussed at IAS 2023 and potential roles for broadly neutralizing antibodies (bNAbs).
Noting it is an exciting time in HIV vaccine research, Carl explained that scientists are exploring how to take what we have learned about bNAbs, which prevented the acquisition of some HIV strains, and turn that into an HIV vaccine.
In addition, Carl and Louis discussed how bNAbs are being studied for use in HIV treatment and even, possibly, a cure.
Previous research indicates that bNAbs may also activate other immune cells to help destroy HIV-infected cells.
And a study published in February 2022 identified an additional antiviral activity of bNAbs, which block HIV-1 release by tethering viral particles at the surface of infected cells.
View and listen to their conversation at the International AIDS Society’s 12th Conference on HIV Science in Germany at this YouTube link.
As of July 27, 2023, the U.S. FDA has not approved any HIV vaccine candidate.

Merck today announced positive topline results from two Phase 3 trials evaluating V116, the company's investigational 21-valent pneumococcal conjugate vaccine in vaccine-naïve and previously vaccinated individuals.
In both studies, V116 had a safety profile comparable to the comparator in the studies.
Results from the STRIDE-3 clinical trial demonstrated statistically significant immune responses compared to PCV20 (pneumococcal 20-valent conjugate vaccine) in vaccine-naïve adults for serotypes common to both vaccines as assessed by serotype-specific opsonophagocytic activity (OPA) 30 days post-vaccination.
Positive immune responses were also observed for serotypes unique to V116.
Additionally, results from STRIDE-6 demonstrated that V116 was immunogenic for all 21 pneumococcal serotypes in the vaccine among adults who previously received a pneumococcal vaccine at least one year before the study.
If approved, V116 would be the first pneumococcal conjugate vaccine designed for adults, says Merck.
Dr. Eliav Barr, senior vice president, head of global clinical development, and chief medical officer of Merck Research Laboratories, said in a press release on July 27, 2023, "These results support the potential for V116 to become an important new preventative option for adults, regardless of prior pneumococcal vaccination status, by expanding coverage to include eight serotypes not currently included in any licensed vaccine."
According to pre-pandemic 2019 U.S. CDC data, the 21 serotypes covered by V116 are responsible for 85% of invasive pneumococcal disease in individuals 65 and older.
V116 includes eight serotypes not currently covered by approved pneumococcal vaccines.
Serotypes unique to V116 include 15A, 15C, 16F, 23A, 23B, 24F, 31, and 35B, which were responsible for approximately 30% of invasive pneumococcal disease in individuals 65 and older, based on pre-pandemic 2019 U.S. CDC data.



