Search API
The UK Health Security Agency (UKHSA) today published Research and Analysis, Mpox outbreak: epidemiological overview, as of August 3, 2023.
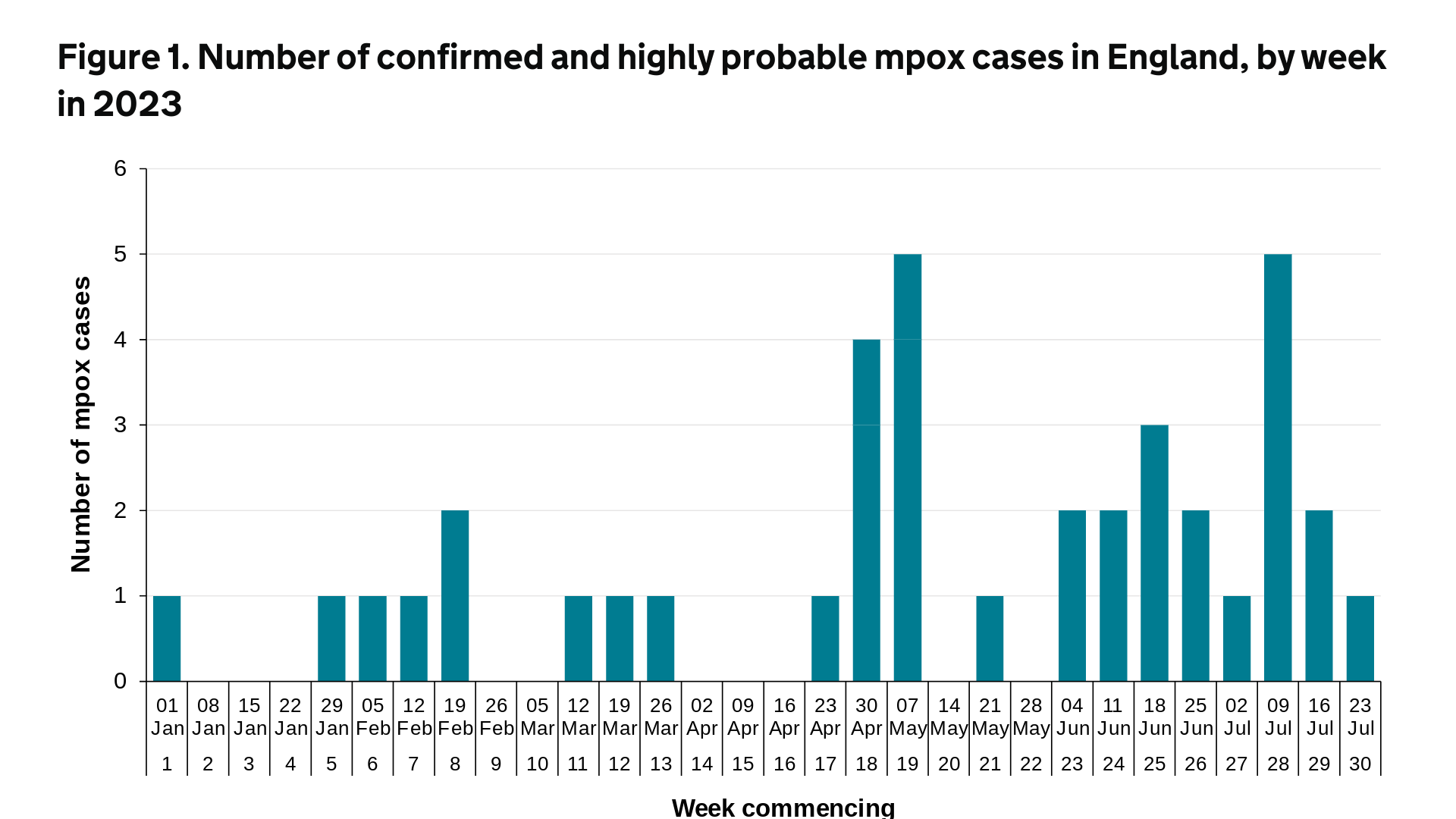
Up to December 31, 2022, there were 3,732 confirmed and highly probable mpox cases reported in the UK. Of these, 3,553 were in England, 34 were in Northern Ireland, 97 were in Scotland, and 48 were in Wales.
In 2023 (up to July 31, 2023), a further 39 cases of mpox were reported in the UK.
Of these, 38 were in England. The most recent mpox cases, seen from April 2023 onwards, have been focused in London.
In reaction, mpox vaccinations have been extended in London because of a spike in infections, says the UKHSA.
The leading mpox vaccine is Bavarian Nordic's JYNNEOS® (MVA-BN) vaccine, which is based on a live, attenuated vaccinia virus.
Bavarian Nordic A/S today announced that the U.S. Biomedical Advanced Research and Development Authority (BARDA) placed a new order valued at USD 120 million, primarily covering the manufacturing of new bulk product for the Company's JYNNEOS® smallpox/mpox vaccine.
The bulk product, representing USD 96 million of the contract value, will be manufactured and invoiced in 2023 and will only partly restore the inventory used to manufacture vaccines in response to the global mpox outbreak that began in May 2022.
Nearly 5.5 million JYNNEOS doses have been manufactured for the U.S. government throughout 2022 and 2023, and replenishment of the bulk inventory is necessary to fulfill the Company's long-term commitment to deliver a freeze-dried version of the vaccine for U.S. smallpox preparedness.
In addition, Bavarian Nordic will manufacture and supply additional liquid-frozen vaccine doses in 2023, valued at USD 3 million.
The agreement includes additional services totaling USD 21 million, of which the majority will be received in 2024 and 2025.
Paul Chaplin, President & CEO of Bavarian Nordic, said in a press release on August 3, 2023, "The U.S. government's foresight enabled us last year to rapidly respond to the global mpox outbreak by converting the readily available bulk product into final vaccine dose."
"Together with our U.S. manufacturing partner, we have completed manufacturing all doses ordered by the U.S. government during the mpox outbreak."
"However, maintaining the readiness to respond to future health crises is essential, and this new contract will enable us to deliver on the contract for a freeze-dried version of the vaccine, awarded to us by the U.S. government back in 2017, which aims to strengthen the nation's preparedness against smallpox."
Since 2003, Bavarian Nordic has worked with the U.S. government on the development, manufacturing, and supply of a non-replicating smallpox vaccine. The JYNNEOS (MVA-BN, IMVANEX®) vaccine has been deployed globally since 2022.
In 2023, mpox outbreaks have been reported Africa, the Americas, Chicago, China, Denver, France, Japan, London, New York, Portugal, South Korea, and Spain. Furthermore, mpox breakthrough cases in vaccinated people have been confirmed in 2023.

Merck today announced that the U.S. Food and Drug Administration (FDA) approved an expanded indication for the ERVEBO® vaccine, which is now indicated for preventing disease caused by Zaire ebolavirus in individuals 12 months of age and older.
ERVEBO was previously approved for use in individuals 18 and older.
This Ebolavirus vaccine does not protect against other species of Ebolavirus (Sudan) or Marburgvirus.
As of March 2023, over 500,000 doses of ERVEBO had been delivered to a stockpile administered by the International Coordinating Group on Vaccine Provision.
"Ebola virus disease is contagious and potentially deadly in children and adults. We're proud of the approval of ERVEBO for the prevention of disease caused by Zaire ebolavirus in children as young as 12 months old, which is another milestone in our continued commitment to help address the global health threat caused by Zaire ebolavirus," said Dr. Eliav Barr, senior vice president, head of global clinical development and chief medical officer, Merck Research Laboratories, in a press release on August 3, 2023.
The vaccine's effectiveness when administered concurrently with antiviral medication, immune globulin, and/or blood or plasma transfusions is unknown, and the duration of protection conferred by ERVEBO is unknown.
ERVEBO includes a contraindication for individuals with a history of a severe allergic reaction (e.g., anaphylaxis) to any vaccine component, including rice protein.
The initial Ebola virus disease case first appeared in 1976 in Africa. Since then, numerous outbreaks of Zaire and Sudan have been confirmed.
As of August 3, 2023, the FDA and European Medicines Agency have approved other ebola prevention and treatment products.

The Janssen Pharmaceutical Companies of Johnson & Johnson recently announced the submission of a supplemental New Drug Application to the U.S. Food and Drug Administration (FDA) seeking to expand the indication of EDURANT® to include the treatment of human immunodeficiency virus type 1 (HIV-1) infection in children weighing 10 kg or more.
As of July 28, 2023, a parallel Marketing Authorization application was submitted to the European Medicines Agency to support a type II variation and line extension for expanded pediatric use in Europe.
If the new applications are approved, EDURANT could be administered to younger pediatric patients via standard 25 mg tablets or new 2.5 mg tablets for oral dispersion that were developed to aid administration and weight-adjusted dosing for children.
“We’ve been working to fight HIV for decades and are proud to have helped bring forward nine medicines for people living with HIV,” said Penny Heaton, M.D., Global Therapeutic Area Head, Infectious Diseases and Vaccines, Janssen Research & Development, LLC, in a related press release.
“These filings are the latest example of our longstanding work to make different treatment options available to meet the diverse needs of people living with HIV.”
EDURANT is not a preventive vaccine but is an HIV-1 specific, nonnucleoside reverse transcriptase inhibitor indicated in combination with other antiretroviral agents for the treatment of HIV-1 infection in treatment-naïve patients 12 years of age and older and weighing at least 35 kg with HIV-1 RNA less than or equal to 100,000 copies/mL.
As of August 2, 2023, the FDA has not approved an HIV vaccine.

GlaxoSmithKline Plc filed a lawsuit alleging Pfizer Inc.'s respiratory syncytial virus (RSV) vaccine infringes on four patents for AREXVY™ RSV OA, a single dose, monovalent RSV vaccine.
AREXVY was the first RSV vaccine approved by the U.S. Food and Drug Administration.
Pfizer's RSV vaccine was the second one approved.
Bloomberg Law reported on August 2, 2023, the GlaxoSmithKline Biologicals SA et al v. Pfizer Inc. complaint was filed in the U.S., District of Delaware.
"Upon information and belief, Pfizer knowingly uses GSK's claimed inventions in ABRYSVO™, a bivalent prefusion F subunit vaccine, without permission," wrote GSK.
GSK is seeking a jury trial, monetary damages, and is asking a judge to prevent Pfizer from selling Abrysvo to adults 60 and older in the U.S.
The U.S. Centers for Disease Control and Prevention announced on June 29, 2023, and July 21, 2023, the use of RSV vaccines for people ages 60 years and older, requires shared clinical decision-making.

The U.S. Department of State officially launched the Bureau of Global Heath Security and Diplomacy.
The Bureau’s overarching mission is to fortify the global health security architecture to effectively prevent, detect, control, and respond to infectious diseases, including HIV/AIDS, wrote the Secretary of State on August 1, 2023.
By leveraging and coordinating U.S. foreign assistance, the Bureau aims to foster robust international cooperation, enhancing protection for the United States and the global community against health threats through strengthened systems and policies.
To ensure U.S. leadership is sustained moving forward, the Bureau will provide a unified voice of leadership on global health security and diplomacy, combining strengths, functions, personnel, and resources from various offices.
Ambassador-at-Large Dr. John N. Nkengasong will lead the Bureau.
This new Bureau will seamlessly integrate global health security as a core component of U.S. national security and foreign policy, underscoring the Department of State’s commitment to advancing human health worldwide, wrote the State Department.
The State Department also issued security notices for most countries, found at this link.

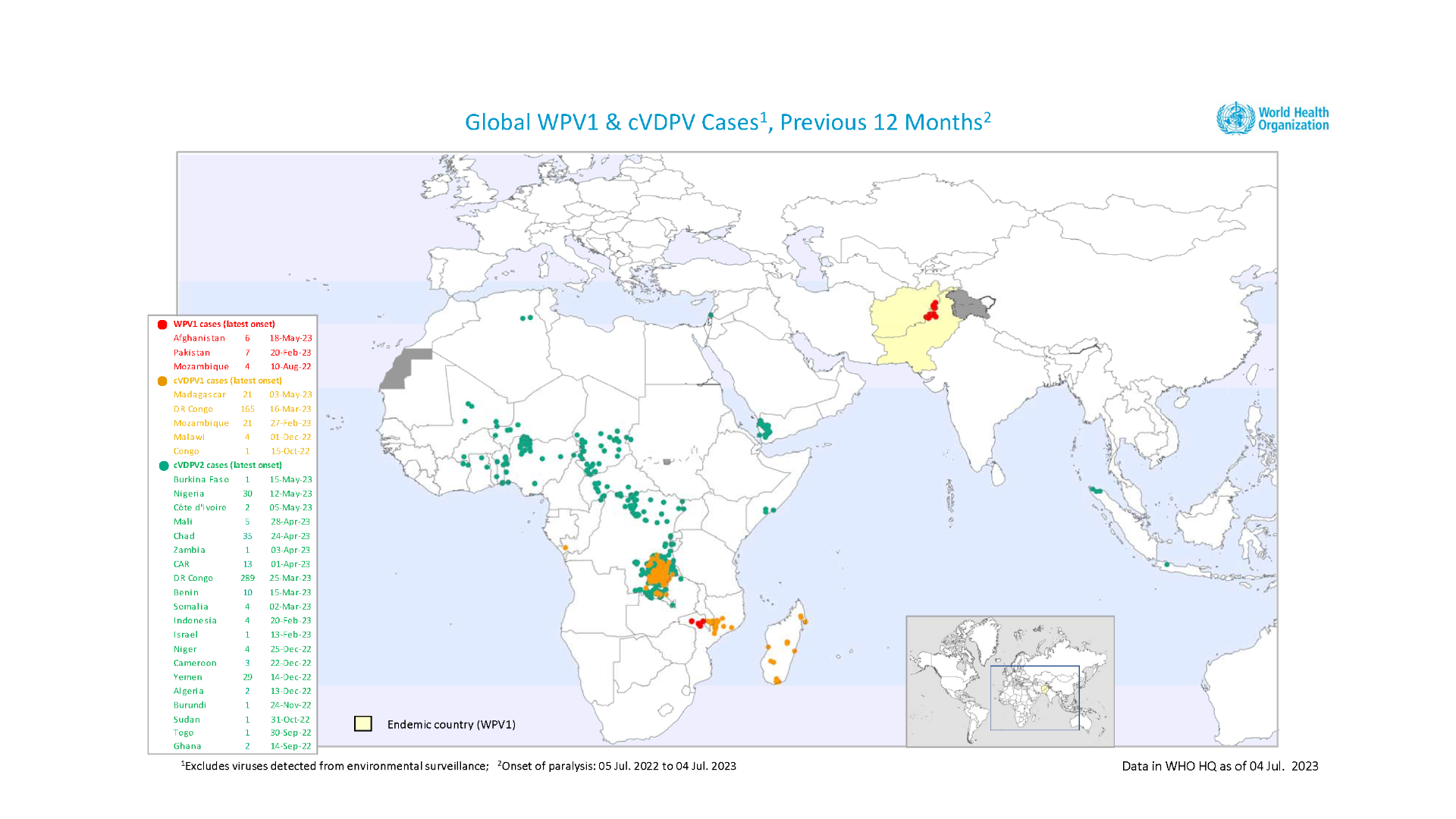
With 117 confirmed cases of circulating variant polioviruses in the WHO African Region this year, the Africa Regional Certification Commission recently urged countries to urgently address gaps in polio immunity to avert future outbreaks.
In recent years, polioviruses have paralyzed hundreds of African children, says the WHO.
On July 28, 2023, the WHO announced the detection of a circulating vaccine-derived poliovirus type 2 (cVDPV2) in two acute flaccid paralysis (AFP) cases and two asymptomatic healthy children community contacts from the Hagadera camp in Kenya.
The U.S. NIH says AFP surveillance is the standard for detecting cases of poliomyelitis in anyone under 15 years of age.
On July 25, 2023, Madagascar launched polio vaccinations for nearly 18 million children, adolescents, and adults in the priority regions of Analamanga, Vakinankaratra, and Alaotra Mangoro. Madagascar has reported 79 cases of cVDPV1 in 2023. Forty-five of them were cases of AFP, including 198 environmental samples.
On July 4, 2023, the Ministry of Health of the United Republic of Tanzania notified the WHO of the detection of cVDPV2. The virus was isolated from an AFP case in the Rukwa region, southwestern Tanzania.
To alert international travelers, the U.S. Centers for Disease Prevention and Control (CDC) included various African countries in its Global Polio Travel Health Advisory on July 10, 2023.
Furthermore, the U.S. was added to about thirty countries where polio was recently identified. In the U.S., poliovirus was confirmed in 2022 and 2023 in wastewater samples.
The CDC recommends polio vaccinations before visiting outbreak areas.