Search API
Bavarian Nordic A/S today announced positive topline results from a Phase 3 clinical trial of its virus-like particle (VLP)-based chikungunya virus vaccine candidate, CHIKV VLP (PXVX0317).
The results up to day 22 post-vaccination showed that CHIKV VLP was highly immunogenic in healthy adolescents and adults, as demonstrated by the strong induction of chikungunya-neutralizing antibodies in 98% of vaccinees in the active group.
The strong neutralizing antibody titres were equal to or exceeded the threshold agreed upon with authorities as a seroprotection marker, meeting the study's primary objectives.
Importantly, CHIKV VLP induced significant neutralizing antibodies in 97% of the subjects at two weeks post vaccination, confirming a rapid onset of protective immunity levels.
These responses were robust and durable, as 86% of the subjects had seroprotective levels of neutralizing antibodies six months post vaccination.
"We are highly encouraged by the positive topline results now demonstrated in both Phase 3 studies of our chikungunya vaccine candidate. Our focus remains to finalize the studies and prepare for regulatory submissions next year," said Paul Chaplin, President and CEO of Bavarian Nordic, in a press release on August 6, 2023.
"With a fast and durable response, our vaccine has the potential to be the best in class to prevent chikungunya infections in adolescents to elderly adults."
"Chikungunya can often result in a severe and incapacitating disease affects large parts of the world, and with international travel on the rise again, our CHIKV vaccine offers a significant opportunity to address this large unmet medical need."
These results help form the basis for submission of a Biologics License Application to the U.S. Food and Drug Administration and a Marketing Authorization Application to the European Medicines Agency in 2024 to support potential launch of the vaccine in 2025.
The U.S. CDC published a Morbidity and Mortality Weekly Report (MMWR) that concluded post-authorization safety data after receipt of a primary Novavax COVID-19 vaccine dose is limited by the low number of doses administered (0.01% of total COVID-19 vaccines administered), available data are consistent with those from preauthorization clinical trials.
And no new safety concerns were identified, wrote the CDC on August 4, 2023.
This MMWR stated from July 13, 2022–March 13, 2023, a total of 69,227 Novavax doses were administered to persons in the U.S., and 230 reports of adverse events after vaccination were received by the Vaccine Adverse Event Reporting System (VAERS).
Among the 230 reports received, 19 (8.3%) were classified as serious, and no deaths were reported after vaccination.
Serious reports included one case of thrombosis, two of pericarditis, one of Guillain-Barré syndrome, and two of seizure.
The remaining serious reports described chest pain, arrhythmia, sickness, hospitalization, adverse event not otherwise specified, balance disorder, peripheral neuropathy aggravated, and vaccine failure.
Limitations of this analysis include reporting biases and inconsistency in the quality and completeness of reports to VAERS.
Furthermore, VAERS data generally cannot be used to determine whether a vaccine caused an adverse event.
In addition, approximately one-half of the reports representing adverse events of special interest lacked medical records for CDC review.
Novavax COVID-19 Vaccine Adjuvanted (Nuvaxovid™, CovoVax™, NVX-CoV2373) was the first protein-based vaccine engineered from the genetic sequence of the SARS-CoV-2 beta coronavirus.

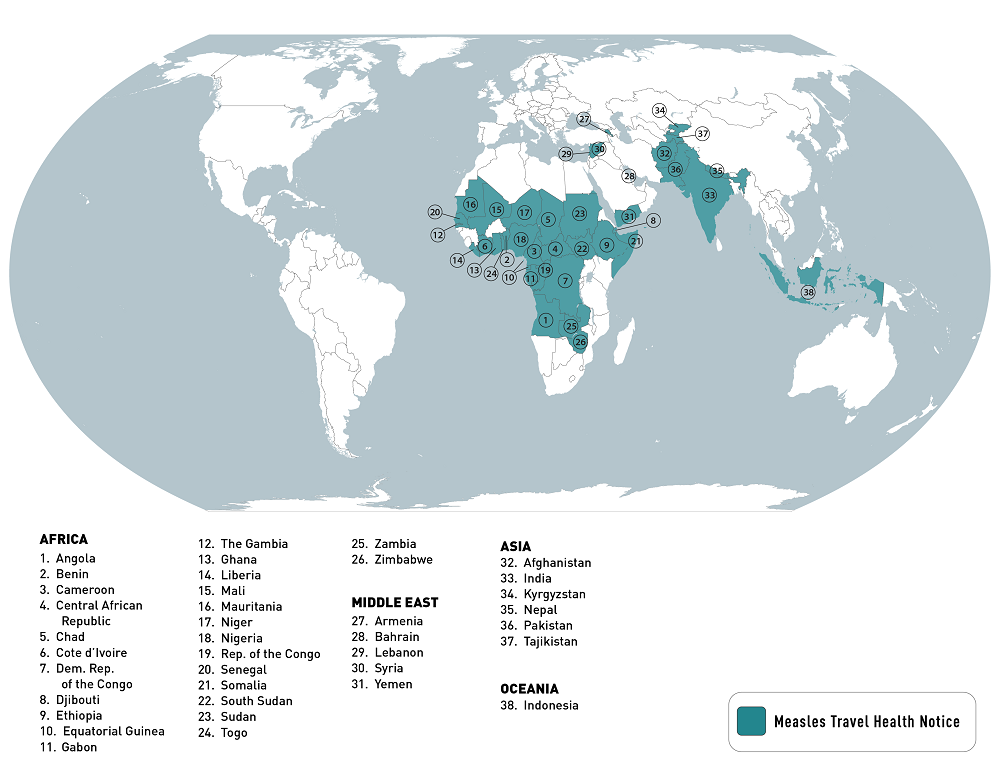
According to the U.S. Centers for Disease Control and Prevention (CDC), measles is an ongoing global health risk.
As of early July 2023, health officials in 38 countries reported large measles outbreaks.
The top ten measles outbreaks were led by India, with 67,592 cases, as of July 18, 2023.
The CDC reissued a Level 1, Practice Usual Precautions Travel Health Advisory to alert international travelers of this risk. The CDC says measles is a risk if a person is not fully vaccinated two weeks before departure or has not had measles in the past.
The CDC says international travelers, including infants 6–11 months of age and preschool-aged children, should be fully vaccinated against measles with a measles-mumps-rubella (MMR) vaccine.
This CDC web tool empowers people to determine whether or not they need additional measles vaccination before departure.
MMR vaccines are generally available in health clinics and community pharmacies in the U.S.
Measles is highly contagious, even on airplanes. Travelers should seek medical care if they develop a rash, high fever, cough, runny nose, or red, watery eyes. Vitamin A deficiency is a recognized risk factor for severe measles infections.
Travelers with suspected measles should notify the healthcare facility before visiting so staff can implement precautions to prevent the spread within the facility.
In the U.S., the CDC has reported 19 measles cases in thirteen U.S. jurisdictions as of August 3, 2023. In 2022, there were 121 measles cases.
Every year, rare human infections with zoonotic influenza viruses usually spread in birds and pigs. The U.S. Centers for Disease Control and Prevention (CDC) recently reported the first two human infections with swine flu viruses in 2023.
These infections were caused by two different types of flu viruses that generally spread among pigs, and they occurred in two people who attended various agricultural fairs in Michigan and had exposure to pigs.
These influenza A(H1N2)v virus infections were thoroughly investigated to ensure that such viruses are not spreading in people and to limit further exposure of people to infected animals if infected animals are identified.
As of August 4, 2023, the CDC recommends people take precautions to prevent the spread of swine influenza viruses to people and has guidance for people exhibiting pigs at fairs, people attending fairs, and fair organizers.
Since 2005, over 500 swine influenza infections have been identified in the U.S.
Unlike, avian influenza (Bird Flu), which has recently spread among birds, mammals, and humans, there are no approved swine flu vaccines.
Furthermore, the CDC says the annual 'flu shot' does not prevent swine influenza infections in people.

The U.S. Transportation Security Administration (TSA) recently reported the number of air travelers passing through airport security has returned to pre-pandemic levels in July and early August 2023.
About 2.4 million people are being screened by TSA security each day.
And according to new data from the TSA, screening can be measured in minutes if you are a TSA PreCheck® member.
During July 2023, about 89% of air passengers waited less than 5 minutes to be processed by TSA PreCheck.
TSA PreCheck is a Trusted Traveler Program offering expedited security screening services at 200 airports in the U.S. To learn more about TSA PreCheck, visit the TSA PreCheck page or the TSA PreCheck® FAQ webpage.

Each summer, influenza viruses are detected in both the northern and southern hemispheres. What happens in one hemisphere does not necessarily predict what will happen in the other because influenza viruses evolve and impact populations differently.
While the exact timing and duration of any flu season varies by country, the World Health Organization (WHO) recently reported most detections in late July 2023 are moderate.
The WHO recently published Influenza Update N° 450, indicating some countries in the southern hemisphere reported changes in influenza detections in recent weeks, while others seemed to have already peaked.
And Australia's Department of Health and Aged Care published report No. 8, which stated insufficient information to assess the 2023 influenza season's potential severity comprehensively.
The WHO and the U.S. CDC suggest international travelers speak with a healthcare provider about flu shot options before visiting countries reporting influenza outbreaks in August 2023.
As of August 5, 2023, most health clinics and community pharmacies in the U.S. offer various flu shots targeting 2023-2024 influenza viruses.

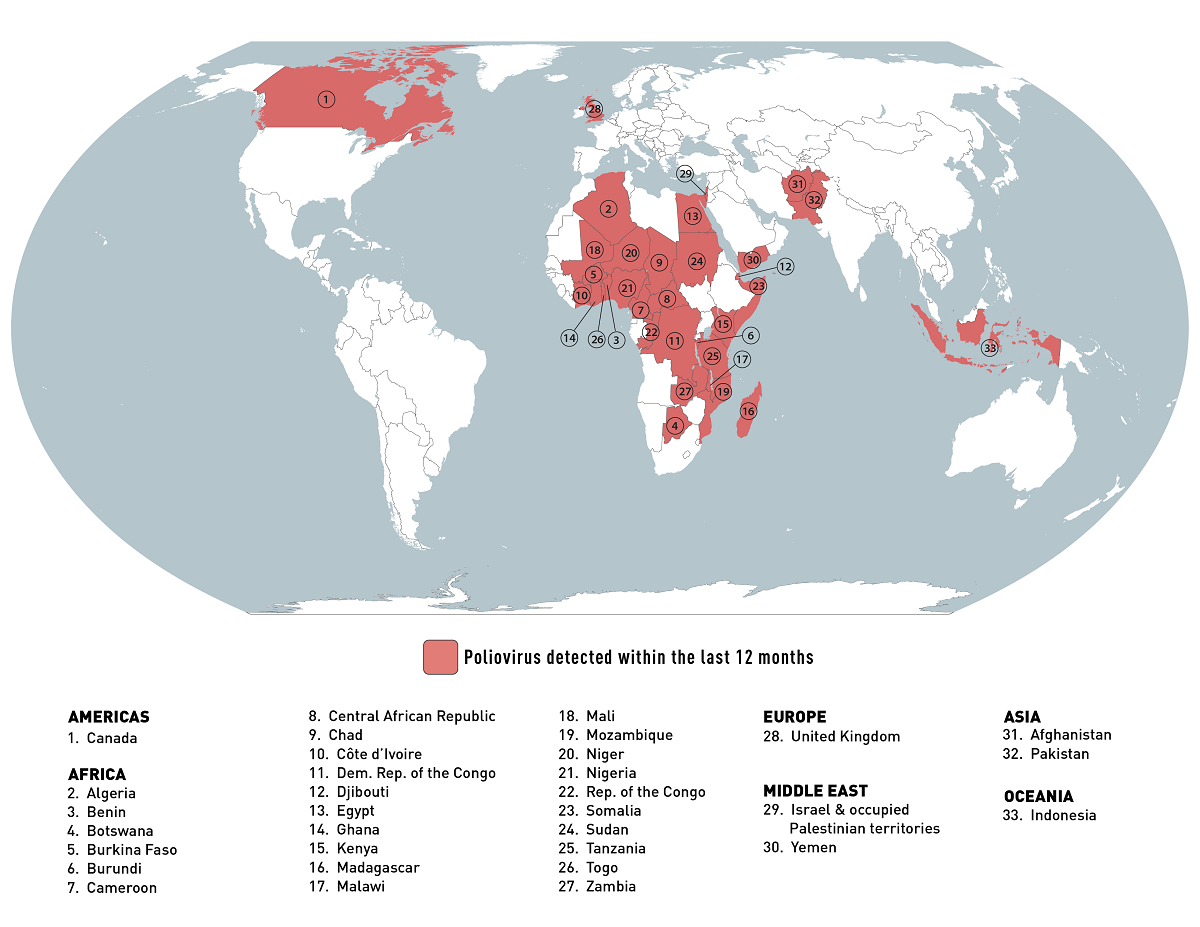
The Global Polio Eradication Initiative (GPEI) reported this week the Democratic Republic of the Congo (DRC) recently confirmed ten new cases of circulating vaccine-derived poliovirus type 1 (cVDPV1).
As of August 2, 2023, there are now 46 cases reported so far this year. There were 146 cases in 2022.
Additionally, the DRC reported four new cases involving circulating vaccine-derived poliovirus type 2 (cVDPV2).
There are now 61 cases so far this year and 367 cases reported in 2022.
In response to this polio outbreak, the U.S. CDC included the DRC in its Level 2 - Practice Enhanced Precautions, Global Polio Travel Health Notice.
The CDC said on July 28, 2023, adults who previously completed the full, routine polio vaccine series may receive a single, lifetime booster dose of an IPV polio vaccine before traveling to any of these 30 destinations.
In the U.S., IPV vaccinations are offered by travel pharmacies.
In the U.S., poliovirus is often detected in wastewater surveillance systems.
Separately, the GPEI published the 6th Transition Independent Monitoring Board report in July 2023, which evaluates the progress and challenges of transferring the responsibility of polio immunization and response efforts to national governments.
The report includes a series of recommendations to strengthen the process at the global, regional, and country levels.