Search API
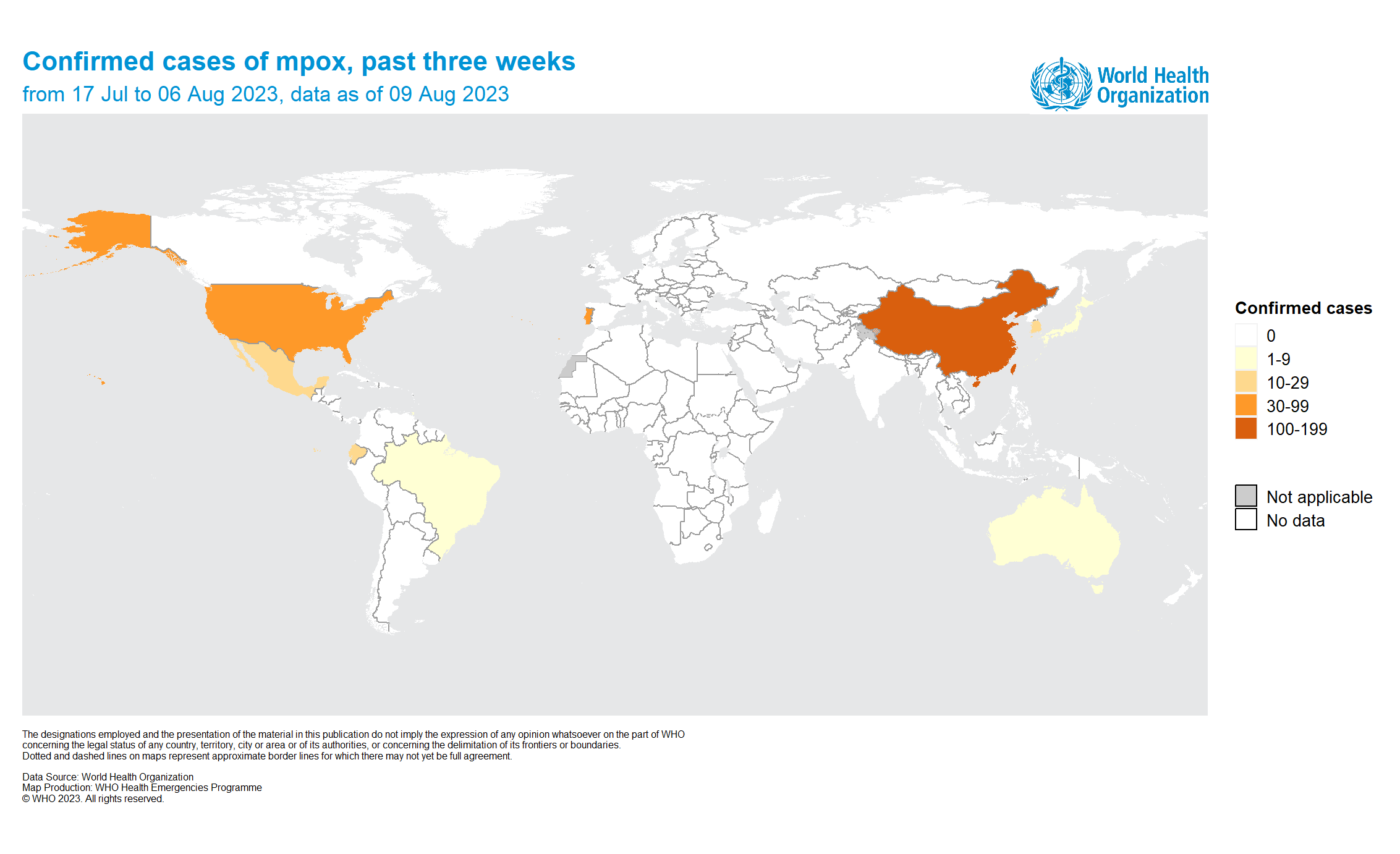
The World Health Organization (WHO) today reported that 15 countries had reported mpox outbreaks in the past three weeks.
As of August 9, 2023, the highest increase in mpox cases was reported in Mexico.
The most affected region was the Western Pacific Region, where 915 cases, the Region of the Americas (395 cases, 9 deaths), and the African Region (227 cases).
The ten most affected countries since May 2022 are the United States of America (30,446), Brazil (10,967), Spain (7,560), France (4,150), Colombia (4,090), Mexico (4,045), Peru (3,812), the United Kingdom (3,771), Germany (3,694), and Canada (1,496).
These countries account for 82.9% of the cases reported globally, says the WHO.
As of August 11, 2023, mpox vaccines remain available in most impacted countries.
The World Health Organization (WHO) today announced the current dengue surge in the Peoples' Republic of Bangladesh is unusual in terms of seasonality and the early sharp increase compared to previous years.
On August 11, 2023, the Ministry of Health and Family Welfare of Bangladesh reported 69,483 laboratory-confirmed dengue cases and 327 related deaths, with a case fatality rate (CFR) of 0.47% during 2023. The CFR so far this year is relatively high compared to previous years for the full-year period.
About 62% of these deaths were reported in July 2023.
Dhaka City Corporation is the most affected area in the Dhaka division, accounting for 78.9% of deaths.
The pre-monsoon Aedes survey shows that the density of disease-carrying mosquitoes and the number of potential hotspots is at the highest level in the past five years.
Globally, dengue outbreaks have been confirmed in numerous countries in 2023.
Dengue virus (DENV) has four serotypes, and infection with one serotype provides long-term immunity to the homologous serotype but not to the other serotypes; sequential infections with a different serotype put people at greater risk for severe dengue.
Many DENV infections produce only mild flu-like illness, and over 80% of cases are asymptomatic.
There is no specific treatment for cases and clinical management is based on supportive therapy.
Neither of the two approved dengue vaccines are currently available in Bangladesh.
The WHO says dengue risk at the national level in Bangladesh is assessed as 'High' due to the ongoing rapidly increasing number of cases and deaths with the peak not yet reached, the high CFR compared to the previous years, and the increasing geographical distribution of cases.

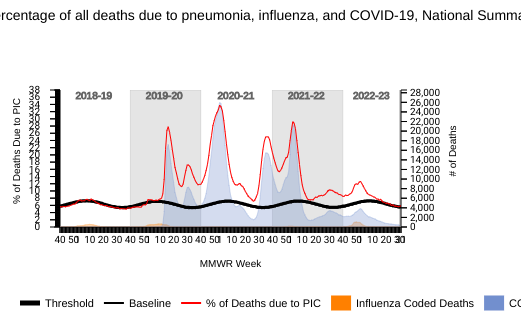
The National Center for Health Statistics (NCHS) Mortality Surveillance data available on August 10, 2023, shows that 5.9% of the deaths during week #31 in the U.S. were due to pneumonia, influenza, and/or COVID-19.
Among these reported deaths, 12 death certificates listed influenza.
Previously, 166 influenza-associated pediatric deaths occurred during the 2022-2023 flu season have been reported.
The recent peak in influenza fatalities was during the 2020-2021 flu season.
The U.S. CDC says these data presented are preliminary and may change as more data are received and processed.
The CDC recommends annual vaccination for most people over six months to mitigate influenza-related fatalities during the 2023-2024 flu season.
Flu is a contagious respiratory illness caused by influenza viruses that infect the nose, throat, and sometimes the lungs.
Most health experts believe that flu viruses are spread mainly by tiny droplets made when people with flu cough, sneeze, or talk. Recent findings suggest that, on average, about 8% of the U.S. population gets sick from the flu each season.
As of August 11, 2023, various flu shots are offered at health clinics and pharmacies in the U.S.
Vaxxinity, Inc. today announced The Lancet's eBioMedicine published results of Phase 2a clinical trial stating that UB-311 "was safe and well-tolerated," with early clinical data demonstrating a trend for slowing cognitive decline in mild Alzheimer's disease (AD).
In this 78-week, randomized, double-blind, placebo-controlled, parallel-group, multicenter Phase 2a, Vaxxinity-funded study, UB-311 was reported to elicit a robust, rapid, and titrated antibody response to Aβ.
And UB-311 was generally well-tolerated, with no cases of ARIA-E and limited cases of asymptomatic ARIA-H.
"This publication supports the innovative work that we and our collaborators are conducting to advance UB-311 for the potential treatment, and even prevention, of Alzheimer's disease," said Mei Mei Hu, CEO of Vaxxinity, in a press release on August 10, 2023.
"Imagine expanding the addressable patient population of beta-amyloid immunotherapies by multiple orders of magnitude, potentially over 1,000x, and delivering life-changing medicine at a fraction of the cost. That is our vision for UB-311 and the potential power of active immunotherapies."
AD is the most common form of dementia, is a progressive neurodegenerative disorder that slowly destroys memory and cognitive skills and eventually the ability to carry out simple tasks.
UB-311 is a synthetic, peptide-based active immunotherapy that targets toxic beta-amyloid (Aβ) oligomers and fibrils and oligomers.
Two passive immunotherapies – monoclonal antibodies targeting Aβ – have recently been authorized by the U.S. FDA, validating Aβ as a target for disease-modifying immunotherapies of AD.
However, these passive immunotherapies have been associated with amyloid-related imaging abnormalities (ARIA), which can present as vasogenic edema or sulcal effusion (ARIA-E) or as hemosiderin deposits such as micro hemorrhages and superficial siderosis (ARIA-H).
Although the trial was not powered to make conclusions about efficacy, secondary efficacy outcomes on cognitive, functional, behavioral, and global assessments such as ADAS-Cog, MMSE, ADCS-ADL, and CDR-SB were evaluated.
Trends of slowing disease progression were observed across key cognitive and functional measures for UB-311-treated versus placebo-treated participants over 78 weeks of observation, including a 48% slowing of decline on CDR-SB in the UB-311 quarterly boosting group.
Furthermore, the U.S. FDA-licensed mAbs require IV infusions every two weeks and are priced at $26,500 annually, not including the cost of administering them or monitoring for ARIA.
In contrast, UB-311 has the potential to offer multiple competitive advantages, including lower rates of ARIA-E, improved convenience through less frequent dosing and ease of administration through intramuscular injection, and overall improved accessibility and cost-effectiveness for patients and health systems.
As of August 10, 2023, the U.S. FDA had not approved an Alzheimer's vaccine candidate.

The U.S. Department of State today confirmed visiting the Republic of Fiji is safe, but people should exercise normal precautions.
The State Department disclosed on August 9, 2023, a higher level of caution is suggested when visiting Colo I Suva Forest Park. Be aware of your surroundings and be extra vigilant along the trails when displaying items like jewelry, bags, and cell phones in public.
In July 2023, a security alert notified U.S. citizen victims of sexual assault are encouraged to contact the U.S. Embassy for assistance at + (679) 331-4466.
Fiji is an island country in Melanesia, part of Oceania in the South Pacific Ocean, where over 200,000 people visit annually.
If you decide to travel to Fiji, the no-cost Smart Traveler Enrollment Program is available to receive security messages and make it easier to locate you in an emergency.
From a health perspective, the U.S. CDC suggests visitors to Fiji speak with a healthcare provider about routine and travel vaccines, including the annual flu shot.
The CDC recently (July 2023) reissued a Travel Health Advisory for the Pacific Islands regarding various dengue outbreaks.

Meissa Vaccines announced positive safety and immunogenicity data for MV-012-968, the company's intranasal live attenuated Respiratory syncytial virus (RSV) vaccine candidate.
Meissa is developing MV-012-968 as a needle-free, adjuvant-free vaccine to protect infants and toddlers from the respiratory syncytial virus (RSV).
The company says RSV is the leading cause of infant hospitalization in the United States and is considered a "missing" pediatric vaccine.
The clinical study in RSV-naïve (seronegative) participants between the ages of six and 36 months enrolled 79 participants at multiple sites in the U.S. to evaluate the safety and immunogenicity of MV-012-968 (NCT04909021).
At the highest dose tested, no serious adverse events related to vaccination were reported with no evidence of any lower respiratory tract symptoms, no Grade 2 or 3 fever observed, and low level, transient vaccine shedding detected.
All RSV-naïve infants and toddlers demonstrated a vaccine response to two doses of 107 PFU.
Serum-neutralizing antibody responses to MV-012-968 were robust and comparable to those seen with previous live attenuated RSV vaccine candidates demonstrating high efficacy against medically-attended RSV disease.
Moreover, MV-012-968 demonstrated significantly greater tolerability to date than these previous candidates.
"With this outstanding safety and robust serum antibody response, we believe Meissa's live attenuated vaccine has the potential to be a best-in-class solution to protect infants and toddlers from RSV, and we are now preparing to advance MV-012-968 into a Phase 2/3 clinical trial next year (2024)," said Martin Moore, Ph.D., co-founder and Chief Scientific Officer, Meissa Vaccines, in a press release on August 8, 2023.
"These data also demonstrate the power of our AttenBlock platform to generate live attenuated vaccines with outstanding safety and immunogenicity – something that we've not seen with other platforms."
As of August 10, 2023, the U.S. FDA has approved RSV vaccines for seniors and antibody therapies for infants.