Search API
Australia's Department of Health and Aged Care's report No. 9 recently confirmed some good news regarding the 2023 flu season.
Influenza-like-illness (ILI) activity in the community has stabilized in the last fortnight (2-weeks), while ILI presentations to ASPREN sentinel general practitioners have decreased as of August 6, 2023.
And there is currently not enough information to comprehensively assess the potential severity of the 2023 influenza season at this time, stated this report.
Since seasonal surveillance commenced in April 2023, 173 (7%) ICU admissions and 194 influenza-related deaths have occurred.
Furthermore, of the 2,449 samples referred during 2023, 98% of influenza A(H1N1) isolates, 83% of influenza A(H3N2) isolates, and 99% of influenza B/Victoria isolates characterized were antigenically similar to the corresponding vaccine components.
However, it is too early to assess influenza vaccine effectiveness for this flu season.

The United States has maintained the elimination of measles since 2000. However, measles outbreaks have recently occurred when people travel to and from the U.S., especially when travelers are unvaccinated or under-vaccinated against measles.
The CDC updated its list of the top ten measles outbreaks on August 10, 2023, indicating India has reported over 57,000 measles cases during the past year.
And they reissued a Level 1 Travel Health Notice in late June 2023, confirming a global measles outbreak.
The U.S. Centers for Disease Control and Prevention (CDC) stated it would conduct a Clinician Outreach and Communication Activity (COCA) webinar on August 17, 2023, focused on eliminating measles in the U.S.
This effort requires continued investment in the measles vaccination program,s which are instrumental to achieving elimination.
Additionally, healthcare providers and public health authorities need to remain vigilant to rapidly recognize measles and take steps to mitigate the spread within communities for continued measles elimination. Healthcare providers should consider measles a diagnosis in anyone with a fever (≥101°F or 38.3°C) and a generalized maculopapular rash with cough, coryza, or conjunctivitis who has recently been abroad, especially in countries with ongoing outbreaks.
Furthermore, the CDC urges all healthcare providers to ensure their patients are current on measles, mumps, and rubella vaccination.
During this COCA Call, presenters will discuss the history of measles in the U.S., review clinical presentation and diagnosis of measles infection, review how to report suspected cases to public health agencies and outline recommendations for measles vaccination in the U.S.
When: Thursday, August 17, 2023, 2:00 PM – 3:00 PM ET; Webinar Link: https://www.zoomgov.com/j/1603132944; Webinar ID: 160 313 2944; Passcode: 532989.
In the U.S., various measles vaccines are generally available at health clinics and community pharmacies.

Valneva SE today announced that the U.S. Food and Drug Administration (FDA) has revised the Prescription Drug User Fee Act (PDUFA) action date for the Biologics License Application (BLA) for VLA1553, a monovalent chikungunya virus vaccine candidate.
Valneva is committed to working with the FDA in its ongoing BLA review and potentially delivering the world's first chikungunya vaccine.
The previously communicated end of August PDUFA has been adjusted to the end of November 2023.
Valneva stated on August 14, 2023, the FDA extended the PDUFA date to allow sufficient time to align and agree on the phase 4 program necessary under the accelerated approval pathway.
Furthermore, no additional clinical data have been requested for the FDA approval process.
Juan Carlos Jaramillo, Chief Medical Officer of Valneva, said in a press release, "We appreciate and take pride in the fact that our BLA for VLA1553 if approved, will represent the first vaccine candidate to be approved under the accelerated approval pathway in an outbreak disease, and hence the necessary Phase 4 activities will set a future standard."
The Company reconfirms its previous guidance for potential BLA approval, initial launch, and potential award of a priority review voucher in 2023. This PDUFA extension does not impact Valneva's current regulatory submission in Canada or its planned submission with the European Medicines Agency.
VLA1553 is a single-dose, live-attenuated chikungunya vaccine candidate based on an infectious clone (CHIKV LR2006-OPY1) attenuated by deleting a gene encoding the non-structural replicase complex protein nsP3 protection against various Chikungunya virus phylogroups and strains.
Valneva's VLA1553 vaccine candidate is designed for prophylactic, active immunization against Chikungunya in humans over 1-year-old.
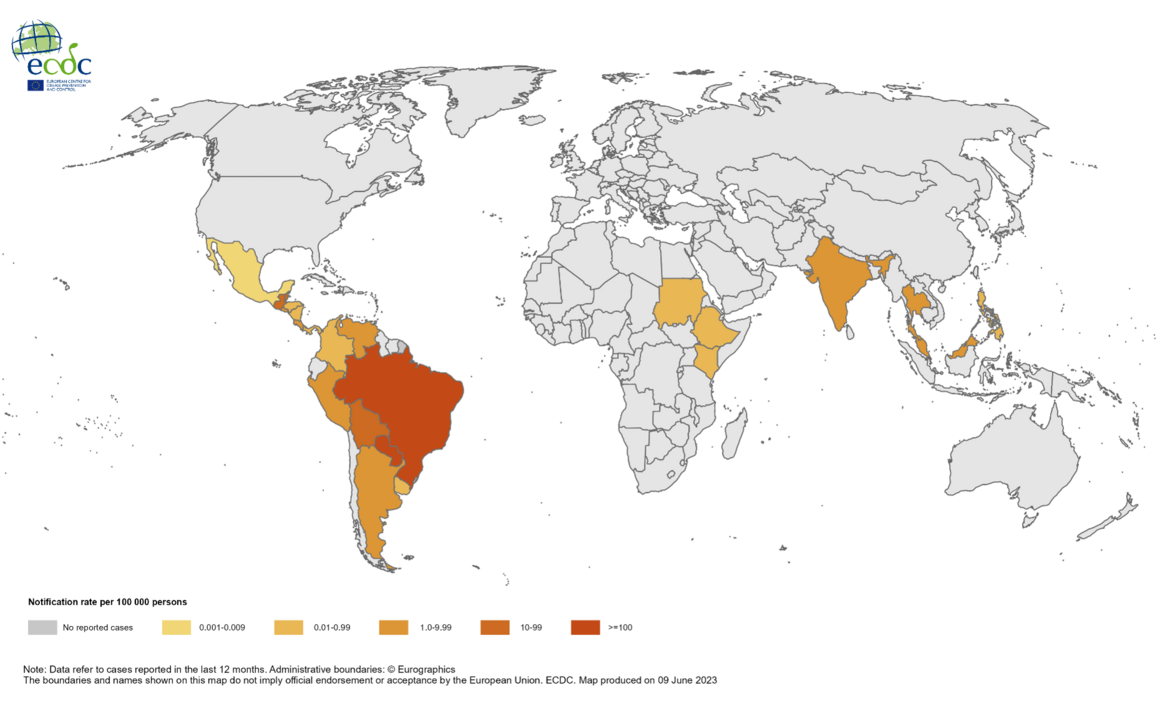
Chikungunya is a viral disease transmitted to humans through the bites of mosquitoes, causing outbreaks in 2023.
The European Centre for Disease Prevention and Control reported that as of July 26, 2023, approximately 300,000 cases and over 300 deaths have been reported worldwide due to Chikungunya virus disease.
Since rabies is a serious ongoing public health concern, the U.S. Department of Agriculture’s Animal and Plant Health Inspection Service (APHIS) announced on August 4, 2023, its annual distribution of RABORAL V-RG®, an oral rabies vaccine (ORV) bait, would be distributed in select areas in the eastern United States to prevent the spread of raccoon rabies.
ORV baits are coated with a fishmeal attractant and are packaged in two-inch plastic sachets or one-inch square cubes.
The RABORAL V-RG vaccine is safe for many animals, including domestic dogs and cats. Humans and pets cannot get rabies from contact with the baits.
If adults or children come in contact with baits, immediately rinse the contact area with warm water and soap, says the APHIS.
While raccoons and dogs are high-risk rabies carriers, wild, infected bats are the leading cause of rabies in the U.S.
Rabies is found in more than 150 countries and territories, say the World Health Organization. And rabies infections are almost always fatal once symptoms appear, but deaths can generally be prevented with appropriate therapies.
The U.S. Centers for Disease Control and Prevention updated its recommendations for rabies preexposure prophylaxis for humans in 2022, now endorsing a two-dose program.
In the U.S., rabies vaccines are available in 2023.

When bringing a dog to the United States in 2023, people should continue to check federal regulations and the recently extended rabies vaccination requirements.
The U.S. Centers for Disease Control and Prevention (CDC) confirmed that effective August 1, 2023, it extended the temporary suspension of the importation of dogs from countries classified as high risk for dog rabies and countries that are not at high risk if the dogs have been in high-risk countries during the previous six months.
However, dogs vaccinated against rabies in the U.S. by a US-licensed veterinarian may re-enter the U.S. from a high-risk country without a CDC Dog Import Permit if the dog:
- has a current, valid US-issued rabies vaccination certificate;
- has ISO-compatible microchip;
- is at least 6 months old;
- is healthy upon arrival; and
- arrives at one of the 18 airports with a CDC quarantine station.
On July 6, 2023, the CDC published the “Notice of Extension of Temporary Suspension of Dogs Entering the United States from Countries with a High Risk of Rabies” in the Federal Register.
According to the CDC, most rabies cases in the U.S. follow bites by bats, not dogs.
An estimated 47,000–55,000 people successfully receive post-exposure prophylaxis each year in the U.S.
Unfortunately, a recent article published in Clinical Infectious Diseases presented the first documented failure of rabies-post-exposure prophylaxis in the Western Hemisphere.
This 84-year-old man died in 2021 about six months after a rabid bat bit his hand.

The United States IHR National Focal Point recently informed the PAHO/WHO of the first human infection with a novel influenza A(H1N2) variant virus (swine flu) identified in 2023.
According to the IHR announcement, a human infection caused by a novel influenza A virus subtype is an event that has the potential for high public health impact.
On July 29, 2023, the person sought medical care at an emergency department in Michigan, and an upper respiratory tract specimen tested positive for influenza A virus on the same day. Later, the patient received influenza antiviral treatment (Oseltamivir).
An investigation by local public health officials identified swine exposure by the patient at an agricultural fair in late July.
No person-to-person transmission of influenza A(H1N2)v virus associated with this case has been identified, and no additional cases of human infection with A(H1N2)v virus have been identified as of August 10, 2023.
The WHO stated in a media release it does not advise special traveler screening at points of entry or restrictions about the current situation of influenza viruses at the human-animal interface.
For recommendations on safe trade in animals and related products from countries affected by these influenza viruses, refer to WOAH guidance.
Since 2005, there have been 512 influenza A variant virus infections (all subtypes), including 37 (human infections with influenza A (H1N2)v viruses reported in the U.S.
As of August 13, 2023, the U.S. FDA has not approved a swine flu vaccine.

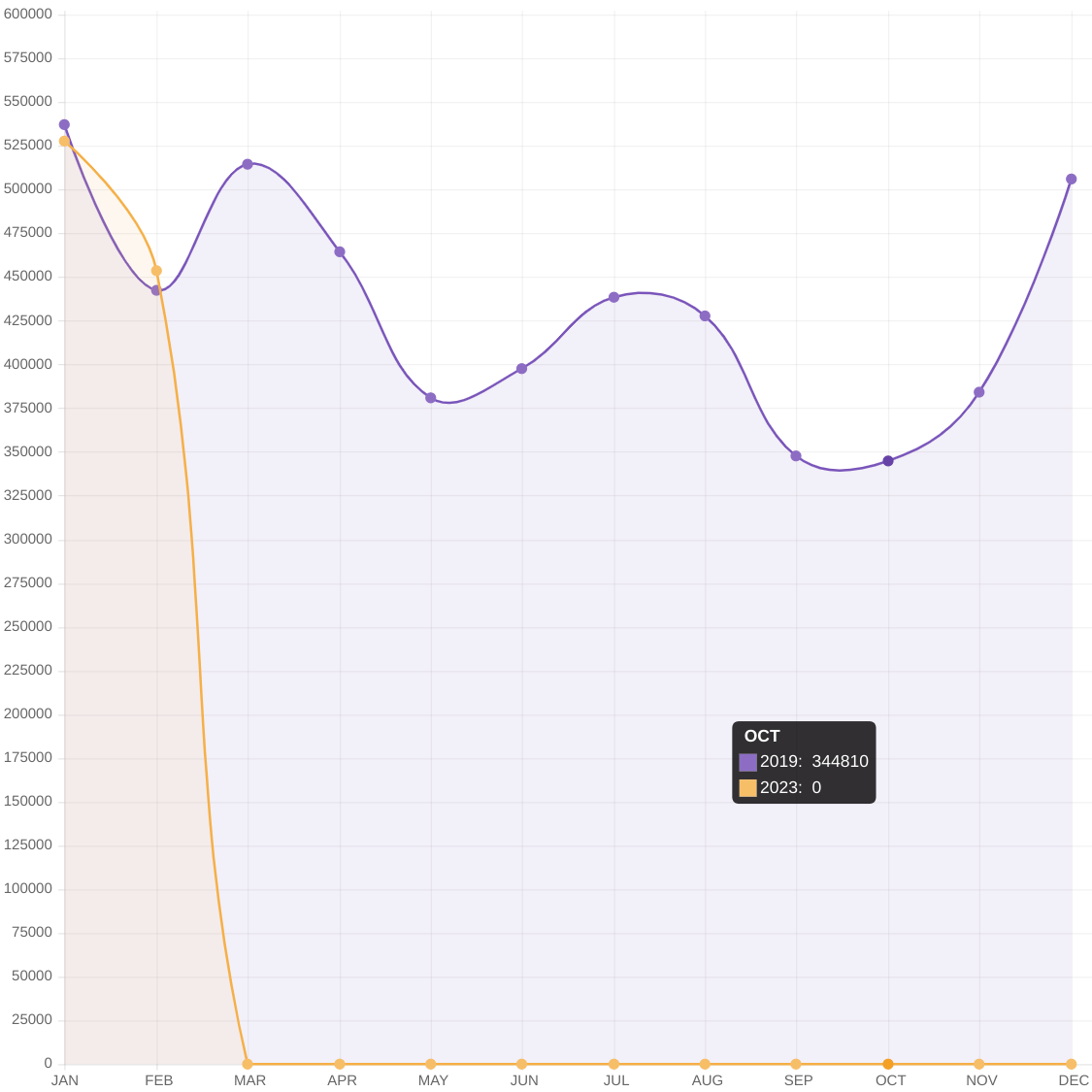
The Republic of Costa Rica's Juan Santamaría International Airport recently reported over 2.8 million passengers passed through its facility during the first half of 2023.
As of June 2023, this activity represents a 4.3% increase in arriving and departing travelers compared to 2019.
The Tico Times reported on August 11, 2023, "The airport now hosts twenty-five airlines serving thirty-five destinations, enhancing global connectivity," said Erick Barboza, AERIS Business Development Director.
Recent AERIS analyses indicate increased Millennial (42%) and Generation X (32%) travelers to Costa Rica.
While in the U.S., the Transportation Security Administration's latest report indicated airport activity has not increased compared to 2019.
From a health perspective, Costa Rica was confronted with dengue, malaria, and Zika cases in 2023.