Search API
The Government of Hong Kong Special Administrative Region's Centre for Health Protection (CHP) of the Department of Health today announced it is closely monitoring a human case of avian influenza A(H5N6) in the Mainland.
The CHP report on August 23, 2023, says the 27-year-old woman lives in Dazhou, Sichuan, and was admitted for treatment on July 22. This report did not disclose how or where she became infected with 'bird flu' nor the outcome.
On July 23, 2023, the Alert Response Level under the Government's Preparedness Plan for an Influenza Pandemic was activated.
From 2014 to date, 86 human cases of avian influenza A(H5N6) have been reported by Chinese health authorities. A high percentage of infections (52%) lead to death.
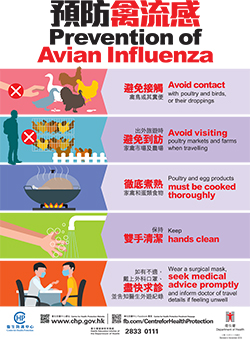
The CHP says that travelers to the Mainland or other affected areas must avoid visiting wet markets, live poultry markets, or farms. And they should strictly observe personal and hand hygiene when visiting any place with live poultry.
The Food and Agriculture Organization of the United Nations, the World Health Organization (WHO), and the World Organisation for Animal Health urged countries in July 2023 to work together across sectors to save as many animals as possible and to protect people.
The current outbreaks of avian influenza ("bird flu") have caused devastation in animal populations, including poultry, wild birds, and some mammals. Although primarily affecting animals, these outbreaks pose ongoing risks to humans, says the WHO.
Since the annual flu shot is not designed to be effective against bird flu viruses, the U.S. government has invested in various avian influenza vaccines.
In June 2023, the U.S. Centers for Disease Control and Prevention confirmed about 20 million H5N1 and 12 million H7N9 vaccines were available in the U.S. National Strategic Stockpile.
The U.S. Administration for Strategic Preparedness and Response's Biomedical Advanced Research and Development Authority (BARDA) today awarded $10 million to Johnson & Johnson Innovation for a competition through project Blue Knight™.
"As the virus continues to evolve, we need new tools that keep pace with those changes," said Assistant Secretary for Preparedness and Response Dawn O'Connell in a press release on August 22, 2023.
This BARDA award is in alignment with 'Project NextGen' which focuses on advancing solutions aimed at addressing health security threats and improving preparedness,
Announced in May 2023, the U.S. Department of Health and Human Services Project NextGen is a $5 billion initiative led by BARDA in partnership with the National Institute of Allergy and Infectious Diseases, coordinates activities across the federal government and the private sector to advance innovative vaccines and therapeutics into clinical trials, regulatory review, and potential commercial availability for the American people.
Announced on May 11, 2023, the Blue Knight challenge offers current and alumni Blue Knight residents and their collaborators the opportunity to apply for the chance to receive funding to help them reach their critical developmental milestones.
Learn more about Blue Knight and hear from current companies at this Johnson & Johnson Innovation LLC link.

Regeneron Pharmaceuticals, Inc. today announced that the U.S. Biomedical Advanced Research and Development Authority (BARDA) entered into an agreement with the Company to support the clinical development, clinical manufacturing, and regulatory licensure process of a next-generation COVID-19 monoclonal antibody (mAb) therapy for the prevention of SARS-CoV-2 infections, which cause COVID-19 in people.
The agreement is part of the U.S. Department of Health and Human Services (HHS) 'Project NextGen' initiative to advance innovative vaccines and therapeutics for COVID-19.
Regeneron's most advanced next-generation antibody candidate under this agreement is expected to enter clinical trials in 2023.
For the new COVID-19 program announced on August 22, 2023, HHS will fund up to 70% of Regeneron's costs for certain clinical development activities for a next-generation mAb therapy.
The new BARDA contract has an estimated value of up to approximately $326 million of government funding.
Regeneron's first COVID-19 mAb cocktail, REGEN-COV, was granted Emergency Use Authorization in November 2020, with nearly 3 million doses delivered to the U.S. Government between 2020 and 2022.
"We're pleased to expand our longstanding BARDA relationship, which is predicated on Regeneron's decades of investment in deep scientific research and enabling technologies," said Leonard S. Schleifer, M.D., Ph.D., Board Co-Chair, President and Chief Executive Officer of Regeneron, in a press release.
"Although COVID-19 has moved to an endemic stage, many people – including those with immunocompromising conditions – continue to face exposure that impacts their everyday life and could cause serious health consequences."
Previously, the U.S. CDC wrote some immunocompromised people benefit from mAb therapy instead of COVID-19 vaccination.
Under the NextGen project structure, Regeneron independently invents and proposes an antibody candidate, which BARDA and Regeneron will evaluate and agree upon for further development, manufacturing, and regulatory activities.
BARDA and Regeneron have previously worked together to deliver novel medicines for Ebola.
The new program announced today falls under Regeneron and BARDA's ongoing Other Transactions Agreement initiated in 2017 to develop a portfolio of antibodies targeting up to ten pathogens that pose significant risks to public health.

In the United States, the timing of seasonal respiratory syncytial virus (RSV) outbreaks throughout the country is generally reported between October - April most years.
The 2022–23 season it started later than in 2021–2022 but earlier than prepandemic seasons, suggesting a return toward normal seasonality.
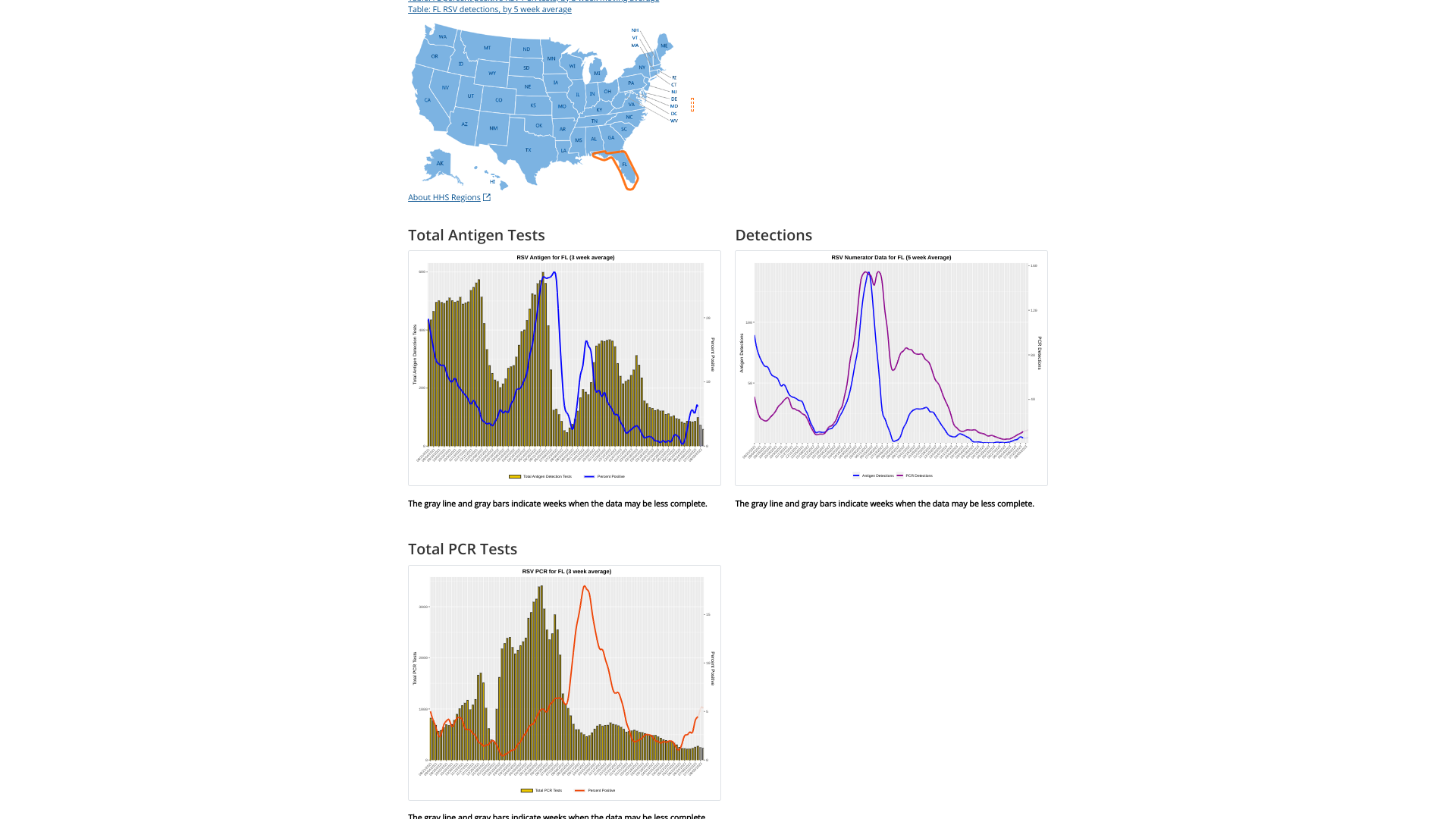
Furthermore, the state of Florida is often a bell-weather state for RSV detections, as it is in 2023.
Florida’s RSV season is longer than the rest of the nation and has distinct regional patterns. For this reason, the state is segmented into five RSV regions, each with its own season.
The Florida Department of Health recently announced RSV activity during week #33 had increasing detection and admission rates.
As of August 19, 2023, two RSV outbreaks were confirmed in Martin County, located north of Jupiter. Last week, one RSV outbreak occurred in Jacksonville, Florida.
In the U.S., RSV infections cause thousands of hospitalizations among young children and adults aged ≥65. The U.S. Centers for Disease Control and Prevention (CDC) RSV detection 5-week moving average graphs for each state are displayed at this link.
This RSV season, the U.S. government has approved RSV vaccines and monoclonal antibody therapeutics available in specific clinics and pharmacies as of August 22, 2023.
The CDC says people should speak with a healthcare provider if these new therapeutics are appropriate for their health.
Novavax, Inc. today announced that its updated protein-based XBB COVID vaccine candidate induced neutralizing antibody responses to the EG.5.1 and XBB.1.16.6 subvariants in small pre-clinical studies.
As of August 22, 2023, SARS-CoV-2 coronavirus XBB sublineage variants are overwhelmingly responsible for the majority of current COVID-19 cases in the U.S. and European Union.
"Our data have shown that Novavax's protein-based COVID vaccine induces broadly neutralizing responses against XBB subvariants, including EG.5.1 and XBB.1.16.6," commented Filip Dubovsky, President of Research and Development, Novavax, in a press release.
Non-clinical data previously showed that Novavax's COVID vaccine candidate induced functional immune responses for XBB.1.5, XBB.1.16, and XBB.2.3 variants, indicating a broad response that could potentially be applicable for forward-drift variants.
Novavax is submitting applications for its XBB.1.5 COVID vaccine candidate to regulatory authorities globally.
Novavax COVID-19 vaccine brands (Nuvaxovid, CovoVax, NVX-CoV2373, TAK-019) have been authorized in about 40 markets.
The Novavax COVID-19 Vaccine, Adjuvanted, has not been approved or licensed by the U.S. FDA but is authorized for emergency use. Novavax vaccines are available in specific clinics and pharmacies in the U.S.

The U.S. Food and Drug Administration (FDA) today approved ABRYSVO™, the first vaccine approved for use in pregnant women to prevent lower respiratory tract disease (LRTD) and severe LRTD caused by respiratory syncytial virus (RSV) in infants from birth through six months of age.
Abrysvo is approved for use at 32 through 36 weeks gestational age of pregnancy, says the FDA.
Previously, the FDA approved Pfizer Inc.'s Abrysvo in May 2023 to prevent LRTD caused by RSV in individuals 60 and older.
"RSV is a common cause of illness in children, and infants are among those at highest risk for severe disease, which can lead to hospitalization," said Peter Marks, M.D., Ph.D., director of the FDA's Center for Biologics Evaluation and Research, in a press release on August 21, 2023.
"This approval provides an option for healthcare providers and pregnant individuals to protect infants from this potentially life-threatening disease."
Recently, the FDA approved Beyfortus™ for infants born during or entering their first RSV season and for children up to 24 months of age who remain vulnerable to severe RSV disease through their second RSV season.
On July 17, 2023, Beyfortus became the first extended half-life monoclonal antibody offering passive immunization to prevent LRTI caused by RSV.
RSV is a highly contagious virus that causes respiratory infections in individuals of all age groups, causing frequent outbreaks. Most individuals can be expected to be infected with RSV by the time they reach two years of age, says the FDA.
In most parts of the U.S., RSV circulation is seasonal, typically starting in Florida and peaking in the winter.
A study published by PLOS Medicine in July 2023 concluded RSV disease burden is high in the nearly 600 million children under five living in 121 low-income and middle-income countries. The peak age of community-based RSV incidence is 4.8 months.

A recent study concluded the high proportion of children too young to be vaccinated among unvaccinated Invasive meningococcal disease (IMD) cases suggests that starting the vaccination earlier may prevent more of these cases.
The JAMA Network published an Original Investigation on a screening cohort study and matched case-control study on August 18, 2023, which found high effectiveness of a 4-component recombinant protein–based (4CMenB) vaccination and more significant reduction in incidence rate ratios (IRR) for early-start vaccination schedules in preventing invasive serogroup B meningococcal disease.
This case-control study represents the most comprehensive multiregional evaluation of the effectiveness of 4CMenB vaccination in the pediatric population of Italy.
Vaccine effectiveness (VE) data obtained from a large group of serogroup B IMD cases with the simultaneous application of 2 independent computational methods (screening and case-control) are unique to the literature.
And VE was firmly greater than 90% in children old enough to receive the first vaccine dose.
Regional differences in the vaccination schedule allowed population-based comparison of outcomes and confirmed the greater efficacy associated with early-start strategies. At the same time, a lack of protection in the very early months of life was apparent even when starting immunization at age two months, prompting the identification of extended prevention strategies.
Current, population-based evidence about VE and reduction in IRRs associated with 4CMenB has been reported in studies conducted in the UK, Australia, Canada, Portugal, and Italy, with heterogeneous methods and across different healthcare settings and age groups.
The estimates of VE for 4CMenB ranged from 59% to 100% in fully vaccinated cohorts.



