Search API
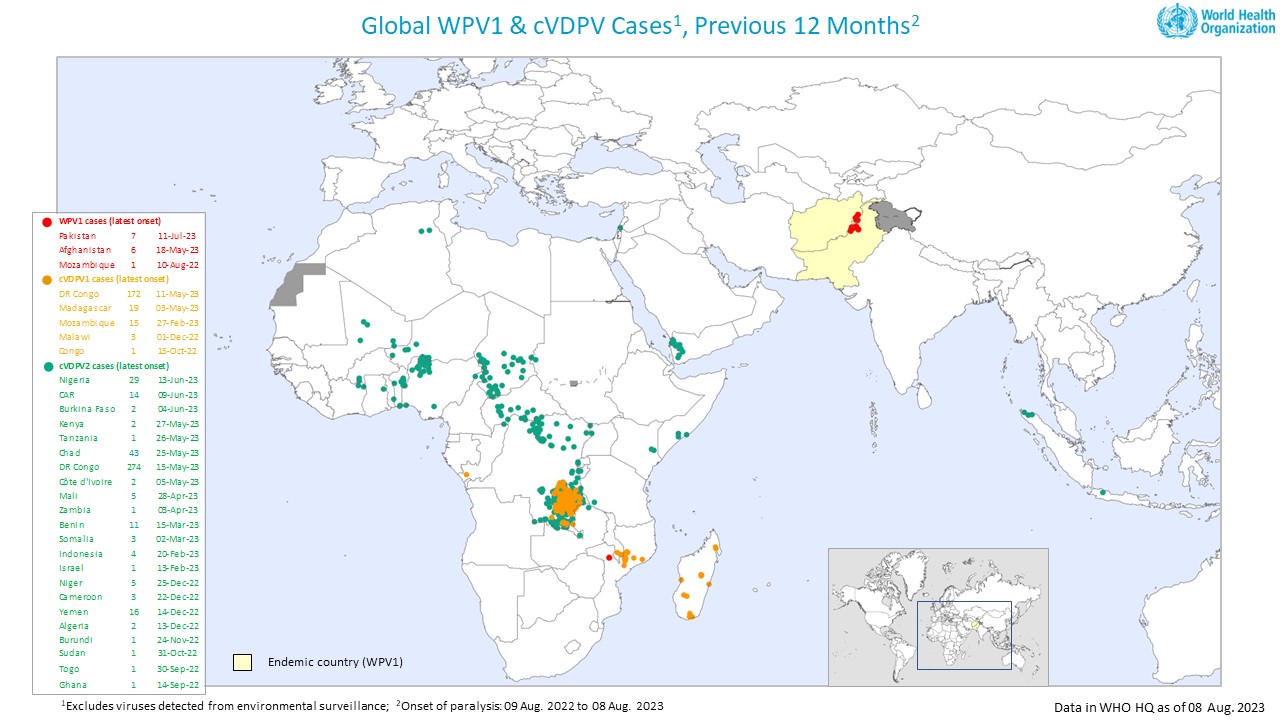
The World Health Organization (WHO) today announced that although encouraged by the reported progress, the Committee unanimously agreed that the risk of international spread of poliovirus remains a Public Health Emergency of International Concern (PHEIC) and recommended the extension of Temporary Recommendations for a further three months.
As of August 25, 2023, only two genetic clusters of WPV1 were identified, compared to three in 2022 and five in 2021.
However, there have been multiple chains of transmission within these two genetic clusters, detected primarily in the endemic zones of Eastern Afghanistan and South KP of Pakistan, including an extreme orphan virus, indicating some gaps in surveillance.
And there is a large pool of unvaccinated "zero dose" children in Afghanistan, which could reintroduce wild-type poliovirus into the southern region.
The also noted suboptimal immunization coverage during campaigns in southeastern Africa, Malawi, Mozambique, Zambia, and Zimbabwe. The nOPV2 vaccine has been deployed over 700 million times in Africa.
Based on the current situation and the reports provided by affected countries, the WHO Director-General accepted the Committee's assessment. It determined that the poliovirus situation continues to constitute a PHEIC.
While poliovirus has been detected in southern New York in 2022 and 2023, no outbreak has been confirmed.
Various polio vaccines are approved and available in August 2023 in the U.S. and globally.
Since the global shortage of oral cholera vaccines (OCV) is forecast to continue until 2025, including in the United States, two companies today announced they are taking action to increase supply.
On August 25, 2023, GC Biopharma confirmed that it signed an MOU with Eubiologics for a co-production of Euvichol®, a World Health Organization (WHO) certified vaccine.
The two companies will coproduce Eubiologics in the first half of 2024 to supply to UNICEF, which has requested an additional supply to cope with the recent spread of cholera infection in many regions, including Africa.
Kyeong-Ho Min, Vice President of Eubiologics, commented in a press release, "With the more frequent floods and droughts due to climate change and global warming, the world is currently experiencing rapid spread of cholera, leading to a shortage of vaccine supply."
Under the MOU, Eubiologics, a developer and producer of Euvichol, takes charge of the bulk vaccine production, and GC Biopharma will be in control of the packaging process, including vial bottling.
Euvichol is an OCV jointly developed by Eubiologics and the International Vaccine Institute that obtained WHO Prequalification in 2015.
Since supplying to UNICEF in 2016, the cumulative supply has exceeded 100 million vaccine doses. Eubiologics is currently providing 100% of cholera vaccines administered by UNICEF.
During 2023, about 49 million OCV doses have been requested, of which 39% were approved for 11 countries, according to WHO report #5.
The WHO stated in August 2023, the current cholera epidemic has deteriorated. Therefore, the WHO assessed the risk of cholera outbreaks at the global level as very high.

The Republic of Finland recently reported another H5N1 avian influenza outbreak at a fur farm involving blue foxes. This outbreak, reported by EuroNews on August 24, 2023, increases the number of 'bird flu' outbreaks at such farms to 23.
In June 2023, several outbreaks of H5N1 were identified among larids in Finland. The first cases in fur farms were detected in July.
Given these findings, the Finnish authorities decided to cull 120,000 foxes and mink on farms affected by the epidemic.
Finland is Europe's leading producer of fox fur, with around 400 fur farms. For example, FashionFinFur is owned by a fur-farming family in Vörå, Finland, that has been In the business since the 1980s.
In the northern hemisphere, bird flu outbreaks among birds, mammals, and people continue in 2023.
As of August 2023, access to bird flu vaccines is controlled by government agencies.
According to recent reports from South America, infectious diseases may continue spreading in late 2023.
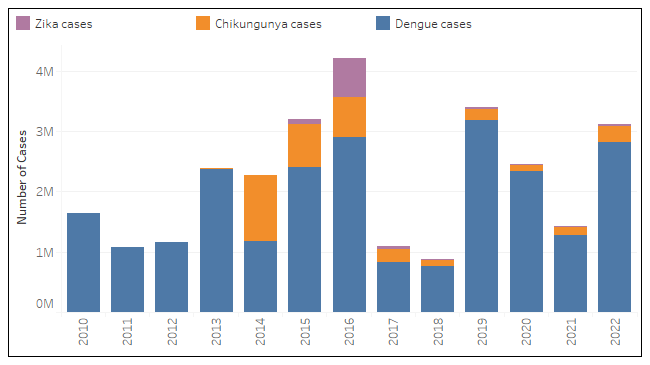
A new study forecasrs a 20% increase in dengue, Zika, and chikungunya cases over the next 30 years. Higher temperatures are already causing the diseases carried by the Aedes aegypti mosquito to spread in cooler regions like southern Brazil and southern Europe.
A study conducted at the University of Michigan concluded after analyzing the incidence of these mosquiuteo-transmitted diseases in Manaus, Recife, Rio de Janeiro, and São Paulo, Brazil.
"Brazilian health agencies need to be prepared not only for the increased incidence of diseases like dengue and Zika but also for longer transmission seasons and broader geographic areas of occurrence," affirms epidemiologist Andrew Brouwer, co-author of the study and researcher at the University of Michigan School of Public Health.
According to Brazil's Center for Arbovirus Emergency Operations, 635 fatal dengue cases had been reported by June 11, 2023, an increase of 22% compared to the same period in 2022.
The agency's most recent update, released by the Ministry of Health, shows 1.3 million probable dengue cases so far this year, while the total number for 2022 was 1,450,270 cases.
The study also showed more potential for Zika epidemics than current levels in all the analyzed climatic scenarios, where the threat had been expected to drop because of extreme heat.
Zika and dengue spread most quickly at average daily temperatures around 30° Celsius, but outbreaks are still possible at 35°C.
As of August 24, 2023, two dengue vaccines are available in certain countries. However, there are no approved Zika vaccines available.
The U.S. Centers for Disease Control and Prevention (CDC) published a Morbidity and Mortality Weekly Report (MMWR) on August 25, 2023, with updated recommendations of the Advisory Committee on Immunization Practices (ACIP) for the 2023–24 Influenza Season.
The primary updates to this new MMWR include the following two topics: 1) the composition of 2023–24 U.S. seasonal influenza vaccines and 2) updated recommendations regarding influenza vaccination of persons with egg allergy.
First, the composition of 2023–24 U.S. influenza vaccines includes an update to the influenza A(H1N1)pdm09 component. U.S.-licensed influenza vaccines will contain HA derived from:
1) an influenza A/Victoria/4897/2022 (H1N1)pdm09-like virus (for egg-based vaccines) or an influenza A/Wisconsin/67/2022 (H1N1)pdm09-like virus (for cell culture-based and recombinant vaccines).
2) an influenza A/Darwin/9/2021 (H3N2)-like virus (for egg-based vaccines) or an influenza A/Darwin/6/2021 (H3N2)-like virus (for cell culture-based and recombinant vaccines).
3) an influenza B/Austria/1359417/2021 (Victoria lineage)-like virus.
4) an influenza B/Phuket/3073/2013 (Yamagata lineage)-like virus.
Second, the ACIP recommends that all persons aged ≥6 months with egg allergy should receive an influenza vaccine.
Any influenza vaccine (egg-based or non-egg-based) that is otherwise appropriate for the recipient’s age and health status can be used.
It is no longer recommended that persons with allergic reactions to eggs involving symptoms other than urticaria should be vaccinated in an inpatient or outpatient medical setting supervised by a healthcare provider who can recognize and manage severe allergic reactions if an egg-based vaccine is used.
Egg allergy alone necessitates no additional safety measures for influenza vaccination beyond those recommended for any vaccine recipient, regardless of the severity of a previous reaction to an egg.
Furthermore, all flu shots for 2023-2024 should be administered in settings in which personnel and equipment needed for rapid recognition and treatment of acute hypersensitivity reactions are available.
A summary of these recommendations is posted on this CDC webpage.

The U.S. Centers for Disease Control and Prevention (CDC) today announced a meeting of the Advisory Committee on Immunization Practices (ACIP) will be held on September 12, 2023, at 10 a.m. ET.
The agenda for this ACIP meeting will include, but is not limited to, a discussion of COVID-19 vaccines and related votes.
For more information on the meeting agenda, visit https://www.cdc.gov/vaccines/acip/meetings/index.html.
The CDC's website publishes Interim Clinical Considerations for the use of COVID-19 vaccines in the U.S., which include a FAQs webpage.
As of August 18, 2023, more than 307 million doses of various COVID-19 vaccines had been administered and reported by the Federal Retail Pharmacy Program. A total of 21 retail pharmacy partners are participating in the program, with more than 41,000 locations nationwide, including long-term care pharmacies.
UPDATED on September 12, 2023 - CDC slide presentations.

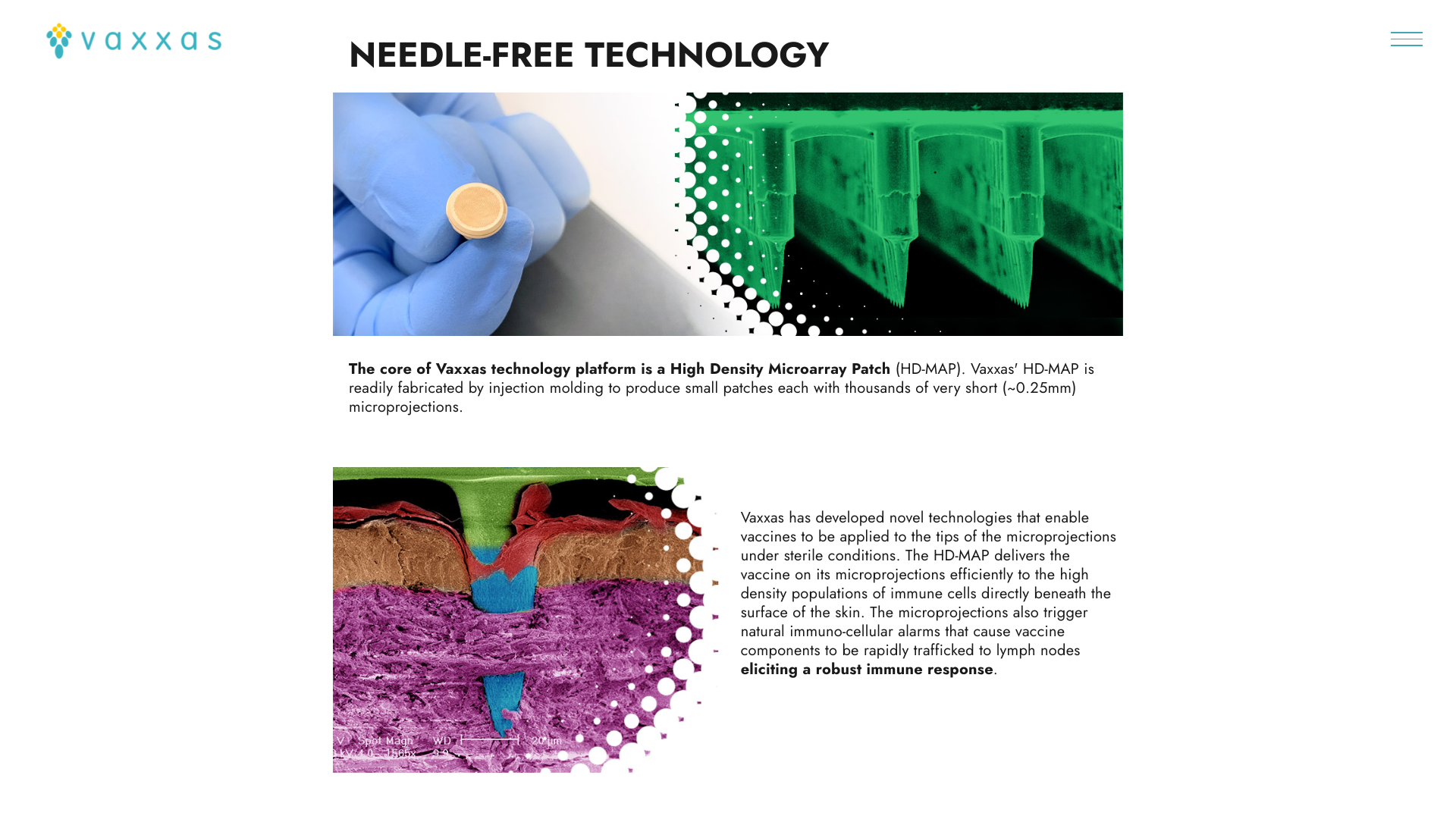
SK bioscience today announced that the company has entered into a collaboration agreement with Vaxxas to develop a second-generation typhoid conjugate vaccine.
SK bioscience’s SKYTyphoid™ vaccine will be reformulated to be ‘printed’ onto the thousands of tiny microprojections covering the Vaxxas patch to be delivered directly to the abundant immune cells just under the skin surface.
This reformulation aims to enhance access and broaden markets where traditional intramuscular delivery using needles and syringes has been employed.
Under the agreement announced on August 23, 2023, SK bioscience will supply the antigen utilized by its typhoid conjugate vaccine, SKYTyhpoid™, jointly developed by SK bioscience and the International Vaccine Institute.
Vaxxas, an Australian company, will be responsible for reformulating the SKYTyphoid antigen so that it can be applied to its proprietary HD-MAPs and then conduct preclinical studies, which, if successful, will be followed by a Phase I human clinical trial.
Jaeyong Ahn, CEO of SK bioscience said in a press release, “Typhoid fever is a dangerous disease that frequently occurs in low- and middle-income countries, but the utilization of typhoid vaccines has been limited due to the requirement for vaccines that remain stable under varying temperatures and those that can be administered without medical supervision."
This collaboration with Vaxxas provides an opportunity to overcome those challenges."
The project is expected to be completed within two years from initiation to reporting the data from the Phase I clinical trial and is supported by grant funding received from Wellcome.
According to the World Health Organization, an estimated 9 million cases of typhoid fever occur globally each year. Symptoms include prolonged high fever, fatigue, headache, nausea, abdominal pain, and constipation or diarrhea, with mortality rates up to 30%.
As of August 24, 2023, typhoid vaccines are available in the U.S. and internationally.
The U.S. Centers for Disease Control and Prevention (CDC) website reported yesterday that a new variant of SARS-CoV-2 betacoronavirus called BA.2.86 was detected in samples from people in Denmark and Israel, and at least two cases have been identified in the United States.
This variant is notable because it has multiple genetic differences from previous versions of SARS-CoV-2.
BA.2.86 may be more capable of causing infection in people who have previously had COVID-19 or who have received COVID-19 vaccines.
Scientists are evaluating the effectiveness of the forthcoming, updated COVID-19 vaccine. CDC’s current assessment is that this updated vaccine will effectively reduce severe disease and hospitalization.
Based on what the CDC knows on August 23, 2023, there is no evidence that this variant is causing more severe illness.
This CDC assessment may change as additional scientific data are developed.