Search API
Vaccines to protect people against Zaire Ebolavirus outbreaks have been used during outbreaks over the past few years.
According to the World Health Organization, two Ebola vaccines are available in 2024.
A recent study has confirmed that the prime-boost Ebola vaccine regimen is safe and effective for children and adults.
This phase 2 study assessed the long-term immunogenicity of the MVA-BN-Filo vaccine regimen and the safety of an immune memory response to an Ad26.ZEBOV booster vaccination.
These researchers concluded, in a paper published on March 26, 2024, that the vaccine regimen and booster dose were well tolerated.
These researchers wrote that a similar and robust humoral immune response was observed for participants boosted one year and two years after the first dose, supporting the use of the regimen and flexibility of booster dose administration for prophylactic vaccination in at-risk populations.
The other recommended Ebola vaccine is Merck's Ervebo®, which was approved in 2019.
However, no vaccines have been approved to protect people against the Sudan Ebolavirus.
In 2024, ten years after the West African Zaire Ebola outbreak, the World Health Organization updated its guidelines on infection prevention and control for Ebola disease.

In 2016, the Philippine Department of Health implemented a dengue vaccination program with a first-generation dengue virus (DENV) vaccine, which was discontinued because of safety concerns.
A recent study assessed the relative risk of developing virologically confirmed dengue among children who did or did not receive a single dose of the Dengvaxia® (CYD-TDV) vaccine by previous DENV infections at baseline classified as none, one, and two or more infections.
This study published by The Lancet Infectious Diseases on March 22, 2024, concluded that a single dose of the Dengvaxia vaccine was ineffective in protecting against DENV among patients who had no prior history of infection or had only one prior infection.
One dose conferred significant protection against hospital admission for virologically confirmed dengue among participants who had two or more previous DENV infections at baseline during the first three years (70%, 95% CI 20–88; p=0·017) and the entire follow-up period (67%, 19–87; p=0·016).
However, young patients exposed to two or more prior DENV infections showed a significant decrease in the risk of DENV infection after receiving the first Dengvaxia dose. This protection continued for up to three years after the vaccination.
Since the study assessed the effect of only a single dose, this study's findings cannot inform public health officers' decisions on vaccination. However, the findings have implications for children who receive an incomplete vaccination regimen, and should prompt more detailed analyses in future trials on dengue vaccines.
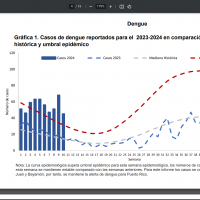
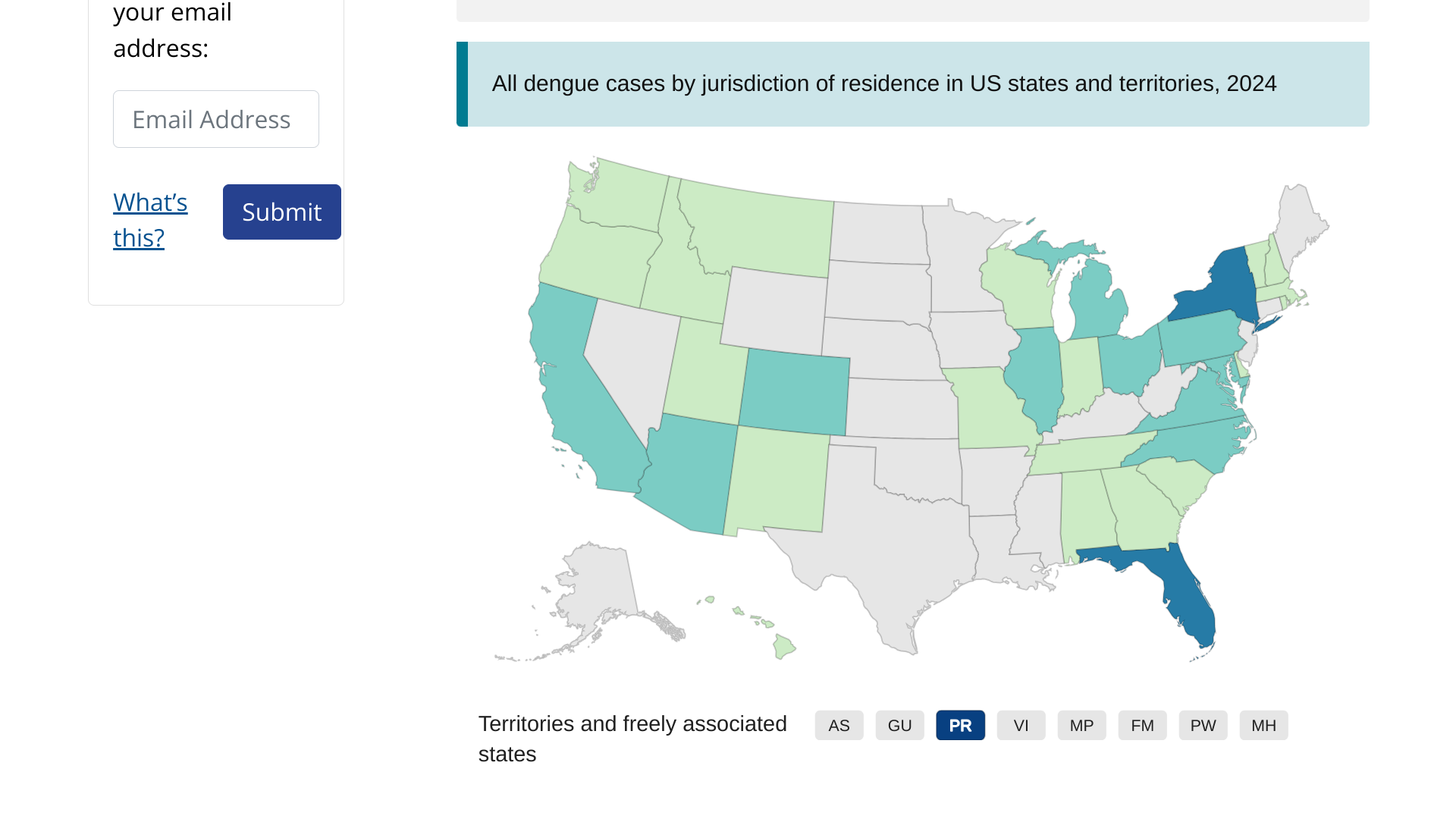
Dengue outbreaks are a global health problem in 2024.
As of March 2024, the CDC has reported over two million dengue cases worldwide, with over 500 deaths. The U.S. CDC has issued a global alert regarding dengue outbreaks in various countries.
A person infected via a mosquito bite will have no symptoms or show clinical manifestations ranging from dengue fever, a mild flu-like syndrome, to dengue shock syndrome, a life-threatening condition.
The CDC recommends speaking with a healthcare provider before visiting dengue-endemic areas like Puerto Rico to discuss vaccination and treatment options.
Blackstone announced today a new collaboration with Moderna, Inc. through a development and commercialization funding agreement which will provide up to $750 million to fund Moderna's influenza vaccine program.
Under the terms of the agreement announced on March 27, 2024, Blackstone Life Sciences (BXLS) will be eligible to receive milestones and royalties on resultant Moderna's influenza products.
Moderna will recognize the funding as a reduction in research and development expenses and will retain full rights and control of the Company's influenza program.
"Moderna has demonstrated a remarkable ability to impact human health through mRNA vaccines targeting respiratory illnesses. This landmark collaboration is another example of our long-standing strategy to partner with the world's leading life science companies to advance their critical path vaccines, medicines, and medical technologies to patients," said Nicholas Galakatos, Ph.D., Global Head of BXLA, in a press release.
Updated information about Moderna's flu program can be found at https://investors.modernatx.com/.
In the United States, flu shot distributions have been decreasing.
As of March 2024, the U.S. CDC reported about 158 million flu vaccines, egg, cell, and nasal-based, had been distributed during the 2023-2024 season. This data compares with 173 million distributed during the 2022-2023 flu season and 194 million during the 201-2022 season.

Defence Therapeutics Inc. today announced the successful testing of a second-generation anti-cancer vaccine, ARM-002TM, using its lead anti-cancer molecule AccuTOX®.
When tested as a therapeutic vaccine in a melanoma cancer model, ARM-002TM led to an 80% complete response when combined with the anti-PD-1 immune checkpoint inhibitor.
The ARM-002TM vaccine was tested in vivo in the context of melanoma.
"AccuTOX® is an amazing molecule! AccuTOX® has the capacity to trigger cancer cell death when used as a direct cancer injectable, and AccuTOX®, the same molecule, converts mesenchymal stromal cells into potent antigen-presenting cells capable of priming potent anti-tumoral responses using 10-fold lower antigen preparation," commented Mr. Sébastien Plouffe, Chief Executive Officer and Director of Defence Therapeutics, in a media statement on March 26, 2024.
Vaccination can stimulate specific immune responses capable of potentially curing established tumors compared to current anti-cancer strategies.
In addition, developed immune cells can lead to a long-lasting memory response capable of further protecting the patient from subsequent cancer relapses, says the company.
According to Data Bridge Market Research, this vaccine segment is expected to reach revenues of about $900 billion by 2029.

BioNTech SE's chief operating officer issued a U.S. Securities and Exchange Commission (SEC) filing on March 22, 2024, confirming that it previously disclosed that the Company was in discussions with the National Institutes of Health ("NIH") concerning royalties and other amounts allegedly owed on sales of the Company's COVID-19 vaccine since commercialization.
The NIH had delivered a communication threatening to send a notice of default under its license agreement with the Company, and the Company received a notice of default from the NIH relating to alleged amounts owed and breaches under such license.
As previously disclosed, the Company disagrees with the positions being taken by the NIH and intends to vigorously defend against all allegations of breach.
BioNTech's recent annual report filing (20-F) with the SEC disclosed an ongoing royalty disagreement with the NIH.
An SEC notice of default informs a contract partner that they have failed to fulfill an obligation and that legal action will be taken if they continue to default.

Osivax, a biopharmaceutical company, today announced that all participants have completed their final visit in the Phase 2a clinical trial (NCT05734040) evaluating OVX836, a broad-spectrum influenza A vaccine candidate, in combination with Quadrivalent Influenza Vaccines (QIVs).
Topline results from this study are expected in the second half of 2024. OVX836 has been tested in 5 clinical trials with 1,200 participants, showing promising safety, immunogenicity, and efficacy read-outs.
OVX836 is a first-in-class influenza A vaccine candidate that targets the nucleoprotein (NP), a highly conserved internal antigen.
Unlike surface antigens, the NP is much less likely to mutate, providing a broader and more universal immune response.
Osivax’s oligoDOMTM technology enables the design and production of a recombinant version of the NP, which self-assembles into a nanoparticle and thus triggers powerful T- and B-cell immune responses.
“The need for more effective and safe flu vaccines remains high, and the conclusion of our trial, which evaluates OVX836 in combination with QIVs, brings us one step closer to providing improved protection,” said Alexandre Le Vert, CEO and Co-Founder of Osivax, in a press release on March 26, 2024.
According to various reports, most flu shots offered about 50% protection during the 2023-2024 influenza season in the U.S.

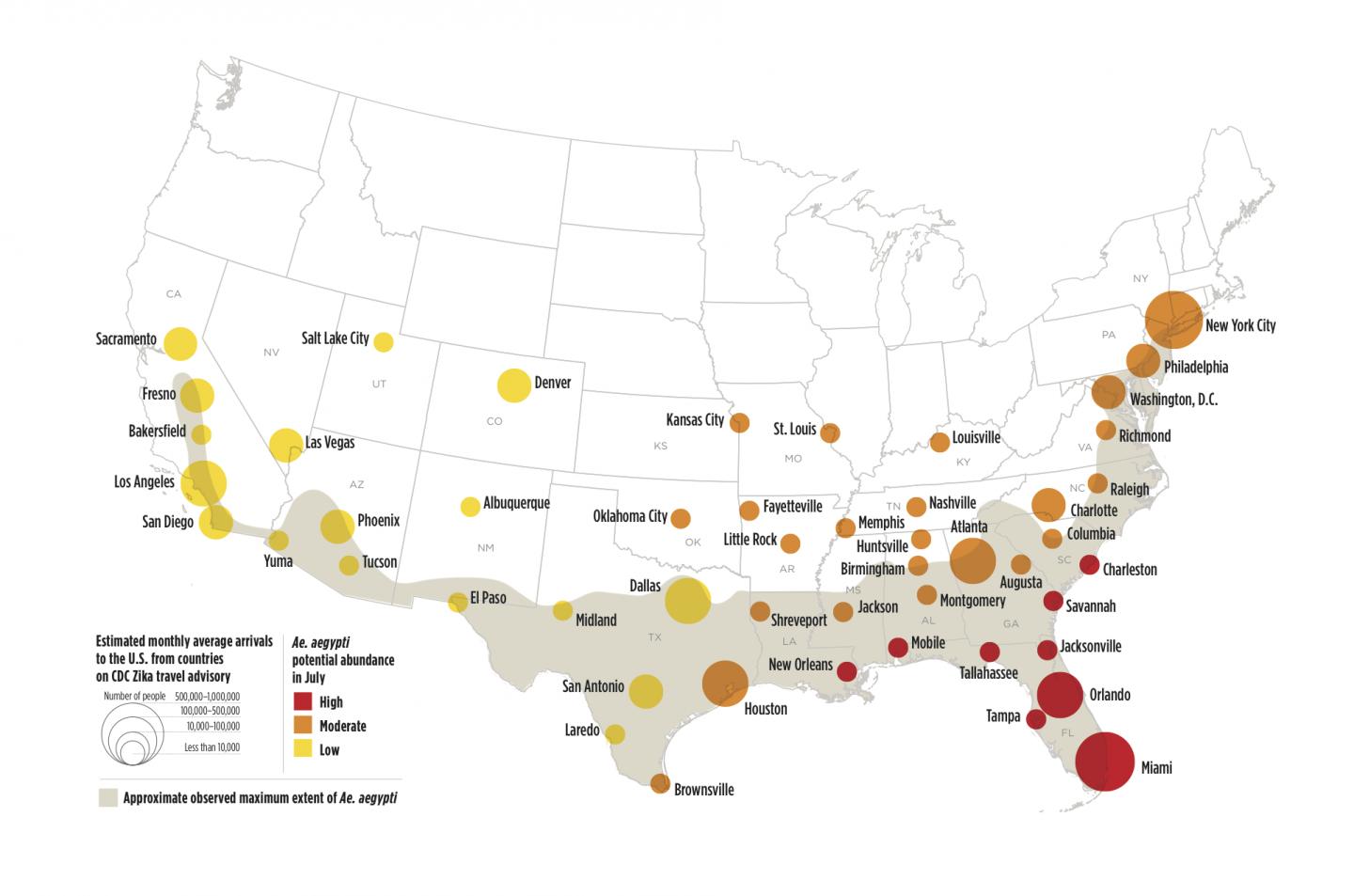
Valneva SE today announced the initiation of a Phase 1 clinical trial to investigate the the safety and immunogenicity of VLA1601, its second-generation adjuvanted inactivated vaccine candidate against the Zika virus (ZIKV).
The randomized, placebo-controlled, Phase 1 trial, VLA1601-102, is planned to enroll approximately 150 participants aged 18 to 49 years in the United States. Topline data from the trial are expected in the first half of 2025.
The initial Phase 1 study results from Valneva’s first-generation Zika vaccine candidate were reported in 2018, showing a favorable safety profile and immunogenicity in all tested doses and schedules.
Juan Carlos Jaramillo, M.D., Chief Medical Officer of Valneva, commented in a press release on March 26, 2024, “Valneva’s commitment to our vision – to live in a world in which no one dies or suffers from a vaccine-preventable disease – fuels our pursuit for preparedness solutions against the Zika virus."
There are currently no preventive vaccines or effective treatments available against ZIKV. However, there are over ten Zika vaccine candidates conducting research.
As such, this mosquito-borne disease remains a public health threat and is included in the Food and Drug Administration’s Tropical Disease Priority Review Voucher Program.
As of March 2024, over 1,100 Zika cases have been confirmed in the Americas this year. Ten countries accounted for about 89% of Zika outbreaks recorded between 2014 and 2023.