Search API
As influenza vaccine producers prepare for the 2024 - 2025 flu season, innovative vaccine candidates are progressing in clinical research, focused on enhancing efficacy and safety.
CureVac N.V. today announced interim data from an ongoing Phase 2 study, which is part of the combined Phase 1/2 study of its seasonal influenza vaccine candidate.
The purpose of this clinical trial (NCT05823974) is to find and confirm the dose and asses the reactogenicity, safety, and immune response of GlaxoSmithKline's (GSK) messenger RNA (mRNA)-based multivalent seasonal influenza vaccine (GSK4382276A) candidates administered in healthy younger and older adults.
Results from the planned interim analysis showed that the multivalent vaccine candidate using CureVac's proprietary second-generation mRNA backbone boosted antibody titers against all encoded flu strains and across all age groups and tested dose levels, including the lowest tested dose.
"The Phase 2 interim data show that CureVac's highly effective and flexible mRNA technology platform puts us on the right track to advance our joint seasonal influenza vaccine program," said Dr. Myriam Mendila, Chief Development Officer of CureVac, in a press release on April 4, 2024.
"Results regarding influenza A strains were strong. Immunogenicity for B strains was also in line with our expectations in view of other initial mRNA-based clinical flu development programs."
"We are confident that planned optimizations will improve performance against these historically challenging influenza strains."
The multivalent candidate was selected from a comprehensive Phase 1 part, which tested vaccine candidates with up to eight separate mRNA constructs per candidate.
It was designed for broad antigen coverage, encoding antigens that matched all World Health Organization (WHO) recommended flu strains.
The WHO says flu shot campaigns should be timed according to local conditions.
Countries are encouraged to analyze local surveillance information to assess their seasonality pattern at both national and subnational levels, as appropriate, to make evidence-based decisions on the timing of vaccination campaigns.
An advanced vaccine candidate for the Lassa fever virus (LASV) today announced the start of its second phase of clinical trials.
This is a significant development, as no approved vaccines for LASV are currently available.
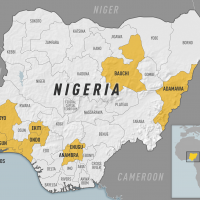
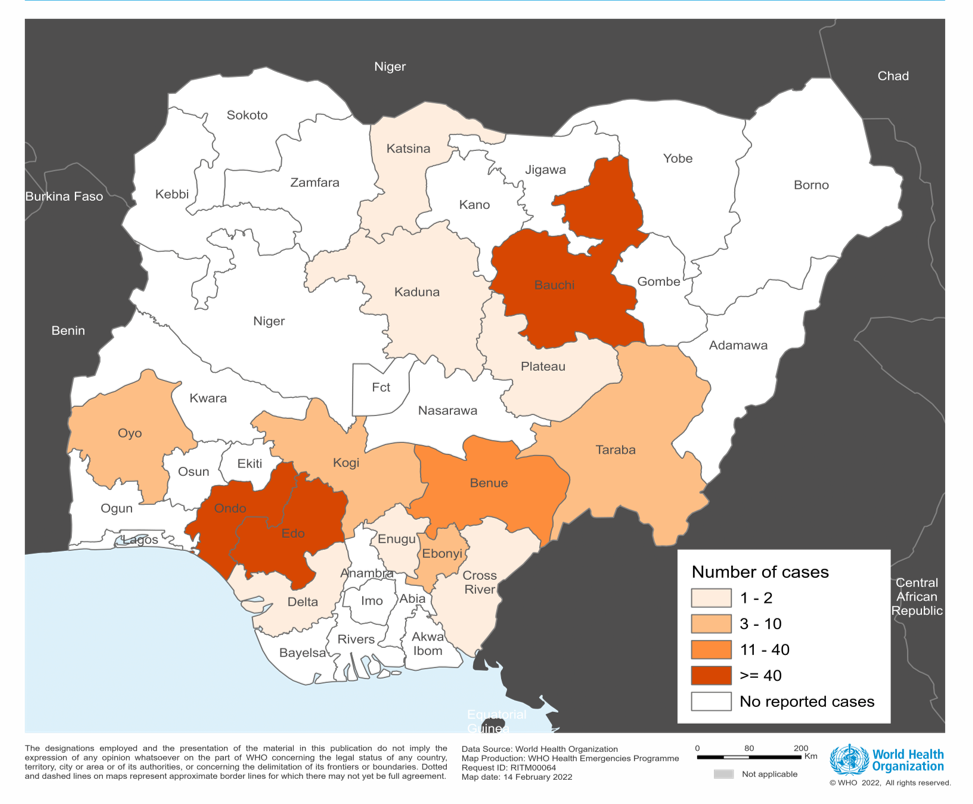
The trial sponsor, International AIDS Vaccine Initiative (IAVI), a nonprofit scientific research organization, confirmed in a press release on April 4, 2024, that participants at HJF Medical Research International in Nigeria had been vaccinated in the first Phase 2 clinical trial of the vaccine candidate rVSV∆G-LASV-GPC.
The IAVI C105/PREVAIL15 study began on March 4, 2024, and is expected to enroll over 600 people in Nigeria, Ghana, and Liberia, with results expected in 2025.
As of March 2024, 27 states in Nigeria have reported at least one confirmed case of LASV.
The vaccine has been developing since 2018 and has been supported and funded by CEPI and the European & Developing Countries Clinical Trials Partnership.
According to IAVI, rVSV∆G-LASV-GPC uses the same recombinant vesicular stomatitis virus vector platform as ERVEBO®, the single-dose Zaire ebolavirus vaccine licensed in North America, Europe, and various African countries.
“Continued outbreaks of Lassa fever and the emergence of Ebola Sudan in Uganda both underscore the need to have vaccines for known disease threats available for evaluation and use during outbreak situations – the overarching goal of IAVI’s emerging infectious disease program,” stated Swati Gupta, DrPH, MPH, vice president and head of emerging infectious diseases and epidemiology, IAVI.
The virus causes acute viral hemorrhagic illness and results in approximately 5,000 deaths and 300,000 illnesses in West Africa each year.
Furthermore, LASV has been included in the World Health Organization's R&D Blueprint of priority pathogens for which accelerated research and development and countermeasures are urgently needed.
In addition to rVSV∆G-LASV-GPC, several other LASV vaccine candidates are conducting clinical research in 2024.
As of April 2024, it remains unclear how long the immune response from mpox vaccination lasts and whether prior smallpox vaccination impacts it.
A recent study assessed the level of antibodies one year after vaccination with JYNNEOS® (MVA-BN®, IMVAMUNE®).
Announced at the European Congress of Clinical Microbiology and Infectious Diseases on March 30, 2024, this abstract indicates that people who had received smallpox vaccination during childhood and had pre-existing immunity showed high levels of antibodies generated by mpox vaccine, which remained high in almost all cases.
The authors suggested in a press release that the decrease in antibodies over time following MVA-BN vaccination may be attributable to its composition.
They stated, "The first and second-generation smallpox vaccines contained replication-competent vaccinia virus. MVA-BN is based on non-replicating virus, which may impact the strength and duration of the immune response, with the advantage of a low risk of side effects."
They add, "Regarding the potential necessity for a booster, it is premature to draw such conclusions. It is unclear how waning antibody levels relate to protection. Immunity also involves other elements, such as T-cell responses."
"Comprehensive clinical monitoring over time, which connects infection rates with antibody levels, is required to make informed decisions about booster vaccination protocols."
Bavarian Nordic A/S, the producer of JYNNEOS, the only FDA-approved mpox vaccine, recently announced that the mpox vaccine is commercially available in the U.S. at clinics and pharmacies.
The standard U.S. FDA regimen for JYNNEOS involves a subcutaneous administration with two injections of 0.5mL four weeks apart.
Note: The study is presented by Ph.D. student Dr. Marc Shamier, Erasmus MC, Rotterdam, Netherlands, from a research team led by Dr. Rory de Vries. No industry conflicts of interest were disclosed.

As polio eradication campaigns are highlighted worldwide, a new collaboration intends to support India's effort to create a polio-free country.
Bharat Biotech, the largest manufacturer of oral polio vaccines, and Bilthoven Biologicals B.V. (BBio), a wholly owned subsidiary of Serum Institute of India Private Limited, announced a collaboration to strengthen the production and supply security of Oral Polio Vaccines (OPV) further.
Through this collaboration, confirmed on April 2, 2024, BBIL and BBio will jointly obtain the regulatory approvals and licenses required to commercially manufacture OPVs in India for global supplies from drug substances manufactured in the Netherlands at BBio.
Dr. Krishna Ella, Executive Chairman of Bharat Biotech, commented in a press release, "Oral polio vaccines have been an integral part of the Govt of India's Universal Immunisation Program for several decades, with Bharat Biotech being one of the largest suppliers to immunization programs across the world."
"This collaboration between BBIL and BBio exemplifies cooperation between vaccine companies, ensuring a secure supply of oral polio vaccines and fortifies the nation's mission to eradicate polio."
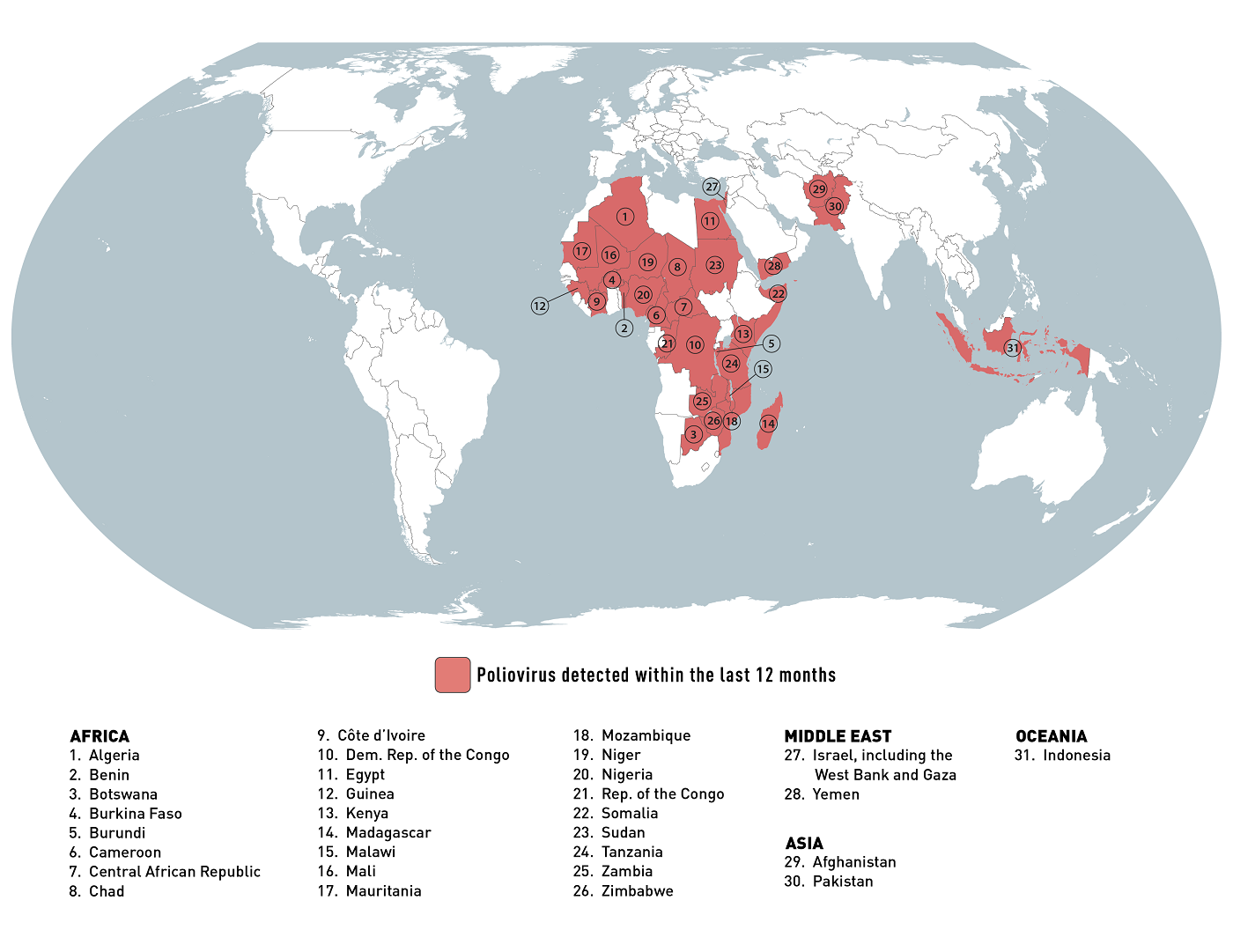
According to the U.S. CDC's Global Polio Travel Health Advisory issued in January 2024, about 31 international destinations are at risk for circulating poliovirus.
The CDC says that before visiting any at-risk destination, adults who have previously completed the full, routine polio vaccine series may receive a single, lifetime booster dose of the polio vaccine.
In the U.S., polio vaccines are offered at clinics and community pharmacies.
Longhorn Vaccines and Diagnostics today announced it is presenting a poster at the World Vaccine Congress on a mice study that examined LHNVD-110, a novel, unconjugated single peptide vaccine candidate comprised of multiple epitopes to broadly target human and influenza viruses.
According to the Company's press release on April 2, 2024, results from the poster show that LHNVD-110 generated broadly reactive antibodies to human and highly pathogenic avian influenza (HAPI) viruses while neutralizing seasonal and pandemic influenza strains.
The Company says using a single peptide provides a more cost-effective and easily scalable approach to such a universal influenza vaccine.
In this study, Longhorn examined mice that were immunized intramuscularly with a low dose (2 µg) and high dose (20 or 40 µg) of LHNVD-110, an unconjugated composite influenza peptide vaccine with multiple highly conserved epitopes of HA, NA, and matrix (M1/M2/M2e), including a universal T cell epitope.
Two booster immunizations were given on days 21 and 35. Isotype specific IgG titers to composite peptides, individual epitopes, and multiple strains of influenza A (H1N1, H3N2, H5N1), and B (Yamagata, Victoria) were analyzed by an enzyme-linked immunosorbent assay, known as ELISA.
This data mirrors previous studies with LHNVD-105, a dual peptide vaccine containing the same epitopes.
"We are studying a single peptide universal influenza vaccine because we believe it could deliver a cost-effective strategy towards formulation and manufacturing and provide immunity against human and avian influenzas," said Longhorn Vaccines and Diagnostics CEO Gerald W. Fischer, MD, in a press release.
"We are expediting IND-enabling studies of LHNVD-110 to prepare for human clinical trials."
The Company wrote, 'While traditional influenza vaccines protect against specific strains predicted for circulation during the upcoming flu season, this often leads to mismatches and variable effectiveness. That is why peptide-based vaccines with broad strain coverage may offer useful strategies for preventing influenza, no matter the strain.'
LHNVD-110 is also formulated with the AddaVax™ adjuvant from InvivoGen.

As the COVID-19 pandemic wanes, upcoming vaccination campaigns will focus on consumer choice in selecting updated vaccines. As of April 2, 2024, the World Health Organization has Listed 13 COVID-19 vaccines.
On April 3, Novavax, Inc. will host a panel discussion, "The Future of COVID-19 Vaccinations," featuring Dr. Robert Walker, the Chief Medical Officer.
This innovative discussion will explore the advantages of creating broadly protective antigens or combination vaccines.
It will showcase continued progress on data from Novavax's updated protein-based COVID-19 vaccine (NVX-CoV2601) with its Matrix-M™ adjuvant and provide an overview of its influenza and COVID-19-Influenza Combination (CIC) vaccine candidates at the World Vaccine Congress 2024 (WVC) in Washington, DC.
New data from Novavax's ongoing research on its updated XBB.1.5 COVID-19 vaccine in participants who previously received an mRNA vaccine showed robust neutralizing antibody titers for the XBB.1.5 subvariant and for the currently circulating JN.1 subvariant. Data also showed that the vaccine's safety and reactogenicity profile was consistent with its prototype vaccine (NVX-CoV2373).
Additionally, Novavax will discuss its influenza and CIC vaccine candidates, including a recap of data to date and the timeline for the Phase 3 trial, which is anticipated to start in the second half of 2024.
COVID-19 vaccines and flu shots are offered at most pharmacies in the U.S.
Furthermore, adults without health insurance and adults whose health insurance does not cover all COVID-19 vaccine costs at an in-network provider can get updated COVID-19 vaccines at a pharmacy for free through the U.S. CDC's Bridge Access Program.

A recent analysis of data from a nationwide health survey reveals "concerning" disparities in human papillomavirus (HPV) vaccine uptake among adults.
More than 84% of the 9,440 people aged 27 to 45 years involved in a national sample published by the journal Human Vaccines & Immunotherapeutics on March 27, 2024, had yet to receive a cancer-prevention HPV vaccine.
The lowest vaccine uptake was observed among men, people of Hispanic heritage, and those with lower educational levels.
Specifically, women had over three times greater odds (aOR = 3.58; 95% CI 3.03, 4.23) of HPV vaccine uptake than men.
Dr. Osazuwa-Peters, who led a team of specialists from institutions, commented in a press release on March 28, 2024, "For oropharyngeal cancer, about 75% of new cases are in males."
"As oral HPV is the primary cause of HPV-associated oropharyngeal cancer, providing the HPV vaccine to middle-aged individuals is undoubtedly an essential strategy for decreasing the risk of infection, persistence, and eventual HPV-associated oropharyngeal malignancy.
"While the population benefit of the HPV vaccines in preventing oropharyngeal cancer may not be realized until years later, there are ongoing clinical trials to establish that the current vaccines are effective in the prevention of oral HPV infection."
HPV is a common sexually transmitted virus that can cause cancers later in life, such as cervical, oropharyngeal, anal, penile, vaginal, and vulvar.
Various HPV vaccines are approved and available worldwide in 2024.